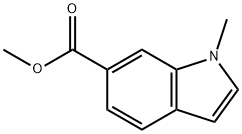
AKOS B006681 synthesis
- Product Name:AKOS B006681
- CAS Number:1204-32-6
- Molecular formula:C11H11NO2
- Molecular Weight:189.21
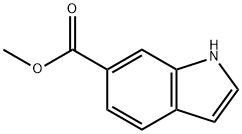
50820-65-0

74-88-4

1204-32-6
Sodium hydride (0.35 g, 8.75 mmol) was slowly added to a stirring solution of methyl 1H-indole-6-carboxylate (0.76 g, 4.34 mmol) in N,N-dimethylformamide (DMF, 10 mL) at room temperature. The resulting suspension was stirred continuously at room temperature for 30 min, followed by dropwise addition of iodomethane (0.54 mL, 8.67 mmol). The reaction mixture was continued to be stirred at room temperature for 20 hours. Upon completion of the reaction, water (20 mL) and ethyl acetate (20 mL) were added to quench the reaction. The aqueous phase was extracted with ethyl acetate (3 x 30 mL), and all organic phases were combined and dried over a phase separation column. Subsequently, the solvent was evaporated under reduced pressure to afford methyl 1-methyl-6-indolecarboxylate as an off-white solid (0.85 g, 100% yield). The product (MH+ = 190) was confirmed by LC-MS (acidic conditions). The purity of the obtained product met the requirements for subsequent use without further purification.

50820-65-0
254 suppliers
$6.00/1g

74-88-4
356 suppliers
$15.00/10g

1204-32-6
77 suppliers
$10.00/250mg
Yield: 100%
Reaction Conditions:
Stage #1:indole-6-carboxylic acid methyl ester with sodium hydride in N,N-dimethyl-formamide at 20; for 0.5 h;
Stage #2:methyl iodide in N,N-dimethyl-formamide at 20; for 20 h;
Steps:
20
To a stirred solution of methyl 1 H-indole-6-carboxylate (0.76g, 4.34mmol) in DMF (10ml) at room temperature was added sodium hydride (0.35g, 8.75mmol). The resultant suspension was stirred for 30min at room temperature followed by the addition of methyl iodide (0.54ml, 8.67mmol). The reaction mixture was stirred for 2Oh at room temperature and was then quenched by the addition of water (20ml) and ethyl acetate (20ml). The aqueous layer was extracted with ethyl acetate (3 x 30ml) and the combined extracts were dried with a phase separating column. The solvent was then removed in vacuo to give the desired product as an off-white solid (0.85g, 100%). LC-MS (Acidic) (MH+=I 90). The product was used without further purification
References:
GLAXO GROUP LIMITED WO2009/71577, 2009, A1 Location in patent:Page/Page column 43
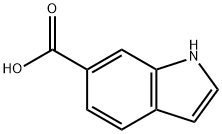
1670-82-2
351 suppliers
$10.00/1g

74-88-4
356 suppliers
$15.00/10g

1204-32-6
77 suppliers
$10.00/250mg
79-22-1
0 suppliers
$38.00/1000g
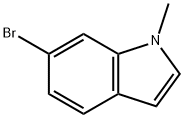
125872-95-9
75 suppliers
$26.00/250mg

1204-32-6
77 suppliers
$10.00/250mg
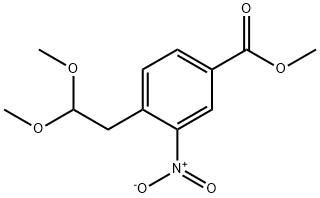
146667-95-0
1 suppliers
inquiry

1204-32-6
77 suppliers
$10.00/250mg
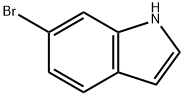
52415-29-9
418 suppliers
$9.00/1g

1204-32-6
77 suppliers
$10.00/250mg