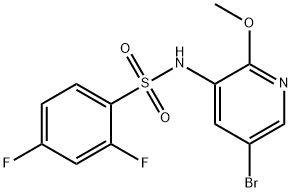
N-(5-bromo-2-methoxypyridin-3-yl)-2,4-difluorobenzenesulfonamide synthesis
- Product Name:N-(5-bromo-2-methoxypyridin-3-yl)-2,4-difluorobenzenesulfonamide
- CAS Number:1086063-46-8
- Molecular formula:C12H9BrF2N2O3S
- Molecular Weight:379.18
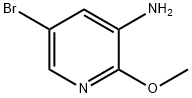
884495-39-0
170 suppliers
$5.00/1g
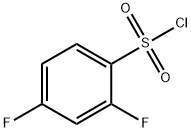
13918-92-8
200 suppliers
$5.00/1g

1086063-46-8
41 suppliers
inquiry
Yield: 89.1%
Reaction Conditions:
Stage #1:5-bromo-2-(methyloxy)-3-pyridinamine;2,4-difluorobenzene-1-sulfonyl chloride with pyridine at 0 - 20; for 19 h;
Stage #2: with sodium hydroxide in methanol at 20; for 12 h;
Steps:
1.3 N-(5-bromo-2-methoxypyridin-3-yl)-2,4-difluorobenzenesulfonamide
To a solution of 5-bromo-2-methoxypyridin-3-amine (10.15 g, 50 mmol) in pyridine (50 mL) was added 2,4-difluorobenzene-1-sulfonyl chloride (11.68 g, 60 mol) slowly at 0° C. The mixture was stirred at rt for 19 hours and concentrated in vacuo. The residue was dissolved in MeOH (100 mL) and NaOH (2.50 g, 60 mmol). The resulted mixture was stirred at rt for 12 hours and concentrated in vacuo. The residue was dissolved in H2O (50 mL) and the resulted mixture was extracted with DCM (100 mL×3). The combined organic phases were washed with brine (100 mL×3), dried over anhydrous Na2SO4, and concentrated in vacuo to give the title compound as a brown solid (16.90 g, 89.1%). [0304] MS (ESI, pos. ion) m/z: 379.0 [M+H]+.
References:
SUNSHINE LAKE PHARMA CO., LTD.;CALITOR SCIENCES, LLC;Xi, Ning US2014/234254, 2014, A1 Location in patent:Paragraph 0303; 0304
67443-38-3
381 suppliers
$5.00/1g

1086063-46-8
41 suppliers
inquiry
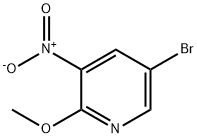
152684-30-5
221 suppliers
$6.00/1g

1086063-46-8
41 suppliers
inquiry
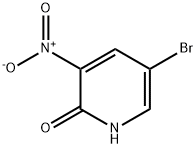
15862-34-7
292 suppliers
$10.00/5g

1086063-46-8
41 suppliers
inquiry