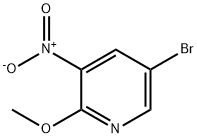
5-BROMO-2-METHOXY-3-NITRO-PYRIDINE synthesis
- Product Name:5-BROMO-2-METHOXY-3-NITRO-PYRIDINE
- CAS Number:152684-30-5
- Molecular formula:C6H5BrN2O3
- Molecular Weight:233.02

67-56-1
67443-38-3

152684-30-5
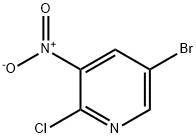
Sodium metal (2.90 g, 126.4 mmol) was added to the cooled methanol (50.0 mL) in batches, and the mixture was slowly warmed to room temperature with continuous stirring until the sodium was completely dissolved. Subsequently, the solution was added to 2-chloro-3-nitro-5-bromopyridine (10.0 g, 42.12 mmol, Shanghai Longsheng Chemical, China) suspended in methanol (100 mL) at 0 °C. The reaction mixture was stirred at 0 °C for 1 hour, then warmed up to room temperature and continued stirring for 16 hours. Upon completion of the reaction, the mixture was concentrated to about 80 mL and the reaction was quenched with deionized water (100 mL). The precipitate was collected by filtration, washed with deionized water (50 mL x 2) and dried under an infrared lamp to afford 5-bromo-2-methoxy-3-nitropyridine as a light yellow solid (9.62 g, 98% yield). Mass spectrum (ESI, positive ion mode) m/z: 233.0 [M + H]+.

67443-38-3
378 suppliers
$5.00/1g

124-41-4
731 suppliers
$12.00/25g

152684-30-5
221 suppliers
$6.00/1g
Yield: 98%
Reaction Conditions:
in methanol at 0 - 20; for 19 h;
Steps:
5.a
a) 5 -bromo-2-(methyloxy)-3 -nitropyridine. To a stirred solution of 5-bromo-2-chloro-3-nitropyridine (86 mmol) in methanol (75 mL) at 0 0C was added drop wise a solution of 25% w/w sodium methoxide in methanol (20 mL, 87 mmol) and methanol (20 mL) over 10 min. After stirring at 0 0C for 1 h the reaction was allowed to warm to room temperature and stirred for 18 h. The reaction was concentrated under vacuum to approximately half its volume then poured into ice water (-500 mL). The precipitate that formed was filtered, washed with cold water, and dried under vacuum to give the title product (98%) as a pale yellow solid. MS(ES)+ m/e 233.2 [M+H]+.
References:
SMITHKLINE BEECHAM CORPORATION WO2009/39140, 2009, A1 Location in patent:Page/Page column 52

67-56-1
790 suppliers
$7.29/5ml-f

67443-38-3
378 suppliers
$5.00/1g

152684-30-5
221 suppliers
$6.00/1g
15862-34-7
290 suppliers
$10.00/5g

74-88-4
357 suppliers
$15.00/10g

152684-30-5
221 suppliers
$6.00/1g

67-56-1
790 suppliers
$7.29/5ml-f

67443-38-3
378 suppliers
$5.00/1g

124-41-4
731 suppliers
$12.00/25g

152684-30-5
221 suppliers
$6.00/1g
13472-85-0
523 suppliers
$8.00/1g

152684-30-5
221 suppliers
$6.00/1g