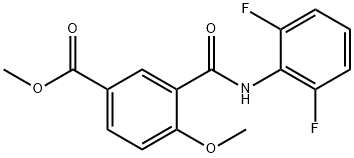
Methyl 3-(2,6-difluorophenylcarbaMoyl)-4-Methoxybenzoate synthesis
- Product Name:Methyl 3-(2,6-difluorophenylcarbaMoyl)-4-Methoxybenzoate
- CAS Number:1089278-51-2
- Molecular formula:C16H13F2NO4
- Molecular Weight:321.28
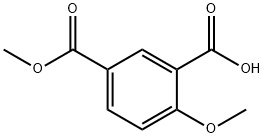
90183-43-0
14 suppliers
$1408.00/500mg
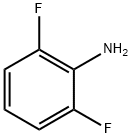
5509-65-9
494 suppliers
$10.00/10g

1089278-51-2
8 suppliers
inquiry
Yield:1089278-51-2 52%
Reaction Conditions:
Stage #1: 2-methoxy-5-(methoxycarbonyl)benzoic acidwith oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane; for 2 h;
Stage #2: 2,6-difluoroanilinewith pyridine;dmap in dichloromethane; for 18 h;
Steps:
2.D
2-(Methyloxy)-5-[(methyloxy)carbonyl]benzoic acid (1.96 g, 9.33 mmol) was suspended in 60 mL of DCM with stirring. DMF (0.036 mL, 0.46 mmol) was added via syringe. Oxalyl chloride (7.0 mL, 2.0M in dichloromethane, 14 mmol) was added dropwise via addition funnel. The addition funnel was rinsed with 10 mL of DCM. The reaction was stirred for 2 hours and concentrated in vacuo. The resultant solid was further dried under high vacuum pressure. The solid was dissolved in 60 mL of DCM with stirring. Pyridine (3.8 mL, 47 mmol), (4-dimethylamino)pyridine (0.0570 g, 0.467 mmol), and 2,6-difluoroaniline (3.0 mL, 28 mmol) were added to the solution. The reaction was stirred for 18 hours and poured into 1N HCl. The layers were separated, and the aqueous layer was washed once with DCM and once with diethyl ether. The combined organic layers were dried over MgSO4, filtered, and concentrated in vacuo. The crude product was purified by flash chromatography. The clean fractions (by TLC) were concentrated in vacuo to afford 1.56 g (52%) of the desired product. 1H NMR (400 MHz, DMSO-d6): δ 9.81 (s, 1H), 8.31 (d, J=2.0 Hz, 1H), 8.10 (dd, J=8.8, 2.0 Hz, 1H), 7.38 (m, 1H), 7.31 (d, J=88 Hz, 1H), 7.22-7.13 (m, 2H), 3.97 (s, 3H), 3.82 (s, 3H).
References:
US2008/300242,2008,A1 Location in patent:Page/Page column 37