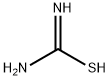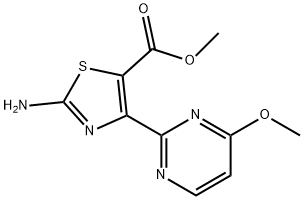
5-Thiazolecarboxylic acid, 2-amino-4-(4-methoxy-2-pyrimidinyl)-, methyl ester synthesis
- Product Name:5-Thiazolecarboxylic acid, 2-amino-4-(4-methoxy-2-pyrimidinyl)-, methyl ester
- CAS Number:1093115-03-7
- Molecular formula:C10H10N4O3S
- Molecular Weight:266.28
Yield:-
Reaction Conditions:
Stage #1: methyl 4-methoxy-β-oxo-2-pyrimidinepropanoatewith N-iodo-succinimide;Amberlyst 15 ion-exchange resin in ethyl acetate at 20; for 1 h;
Stage #2: thiourea in methanol; for 1.5 h;Heating / reflux;
Steps:
71
Methyl 2-amino-4-(4-methoxypyrimidin-2-yl)thiazole-5-carboxylate Methyl 3-(4-methoxypyrimidin-2-yl)-3-oxopropanoate (Intermediate 72; 250 mg, 1.19 mmol) was dissolved in EtOAc (10 ml), Amberlyst 15 ion-exchange resin (230 mg) and n-iodo succinimide (282 mg, 1.19 mmol) were added, and the reaction mixture was stirred at room temperature for 1 hr. The mixture was filtered and the filter cake was rinsed with MeOH, the combined filtrate was concentrated to dryness. Either was added and the resulting precipitate was filtered off, the filtrate was concentrated to an oil and dried under high vacuum. To this crude thiourea (136 mg, 1.78 mmol) and MeOH (10.00 ml) was added and the mixture was refluxed for 1.5 hr, cooled down to rt and yellowish precipitate was filtered and washed with MeOH, the filter cake was washed with saturated Na2CO3 aqueous solution and kept as the desired product (off-white solid), the filtrate was then concentrated to dryness and was suspended into saturated Na2CO3 solution (10 ml), the resulting precipitate was collected by filtration. LC-MS showed both solids were the desired product. (97 mg). MS (ES) (M+H)+: 267 for C10H10N4O3S; NMR (CDCl3): 3.55 (s, 3H), 3.89 (s, 3H), 6.95 (d, 1H), 7.95 (br, 2H), 8.62 (d, 1H).
References:
US2008/312255,2008,A1 Location in patent:Page/Page column 82-83
463337-53-3
16 suppliers
inquiry

1093115-03-7
2 suppliers
inquiry

89640-54-0
6 suppliers
inquiry

1093115-03-7
2 suppliers
inquiry
22536-63-6
180 suppliers
$14.00/1g

1093115-03-7
2 suppliers
inquiry
262353-35-5
74 suppliers
$27.00/250mg

1093115-03-7
2 suppliers
inquiry