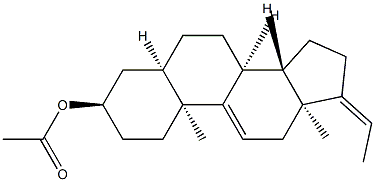
(3R,5R,8S,10S,13S,14S,Z)-17-ethylidene-10,13-dimethyl-2,3,4,5,6,7,8,10,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate synthesis
- Product Name:(3R,5R,8S,10S,13S,14S,Z)-17-ethylidene-10,13-dimethyl-2,3,4,5,6,7,8,10,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate
- CAS Number:1093397-69-3
- Molecular formula:C23H34O2
- Molecular Weight:342.5149
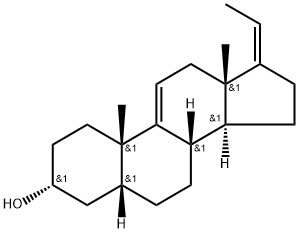
1093397-66-0
14 suppliers
inquiry

108-24-7
0 suppliers
$14.00/250ML
![(3R,5R,8S,10S,13S,14S,Z)-17-ethylidene-10,13-dimethyl-2,3,4,5,6,7,8,10,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate](/CAS/20200331/GIF/1093397-69-3.gif)
1093397-69-3
9 suppliers
inquiry
Yield:1093397-69-3 95%
Reaction Conditions:
with dmap;triethylamine in dichloromethane at 25; for 2 h;Inert atmosphere;
Steps:
16 (Z)-3α-Acetoxy-5β-pregna-9(11),17(20)-diene (7a)
Compound 6 (19.0 g, 63 mmol) was dissolved in CH2Cl2 (380 mL). Triethylamine (17.6 mL, 126.6 mmol), DMAP (0.772 g, 6 mmol) and acetic anhydride (8.98 mL, 94 mmol) were added sequentially at 25 °C under a nitrogen atmosphere. The resulting solution was stirred for 2 h at 25 °C, at which point the reaction was determined by TLC to be complete. The reaction was quenched by the addition of ice-water (100 mL) and the phases were separated. The aqueous layer was extracted three times with DCM (3 × 150 mL). The organic fractions were combined and washed with saturated brine solution (100 mL), dried over anhydrous Na2SO4 (50 g), and filtered. The filtrate was concentrated under vacuum to afford compound 7a (22.0 g, 95% yield) as an off-white solid. Table 21 describes the measured properties of the product.
References:
EP2407475,2015,B1 Location in patent:Paragraph 0123; 0124
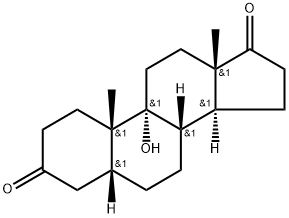
1093397-61-5
1 suppliers
inquiry
![(3R,5R,8S,10S,13S,14S,Z)-17-ethylidene-10,13-dimethyl-2,3,4,5,6,7,8,10,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate](/CAS/20200331/GIF/1093397-69-3.gif)
1093397-69-3
9 suppliers
inquiry
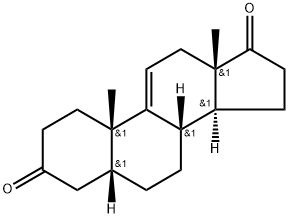
1093397-63-7
13 suppliers
inquiry
![(3R,5R,8S,10S,13S,14S,Z)-17-ethylidene-10,13-dimethyl-2,3,4,5,6,7,8,10,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate](/CAS/20200331/GIF/1093397-69-3.gif)
1093397-69-3
9 suppliers
inquiry
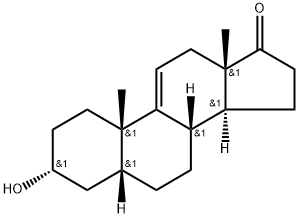
571-49-3
10 suppliers
inquiry
![(3R,5R,8S,10S,13S,14S,Z)-17-ethylidene-10,13-dimethyl-2,3,4,5,6,7,8,10,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate](/CAS/20200331/GIF/1093397-69-3.gif)
1093397-69-3
9 suppliers
inquiry