3-OXOCYCLOHEX-1-EN-1-YL TRIFLUOROMETHANESULFONATE(WXG02961) synthesis
- Product Name:3-OXOCYCLOHEX-1-EN-1-YL TRIFLUOROMETHANESULFONATE(WXG02961)
- CAS Number:109459-28-1
- Molecular formula:C7H7F3O4S
- Molecular Weight:244.19
Yield:-
Reaction Conditions:
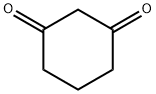
Stage #1:1,3-cylohexanedione with sodium hexamethyldisilazane in tetrahydrofuran at -50;
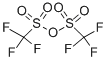
Stage #2:trifluoromethanesulfonic acid anhydride in tetrahydrofuran at -50 - 20;
Stage #3: with water;sodium hydrogencarbonate in tetrahydrofuran at -30;
Steps:
STEP 1: 3-OXOCYCLOHEX-1-ENYL TRIFLUOROMETHANESULFONATE A 1.0M solution of sodium bis(trimethylsilyl)amide in THF (102 mL, 102 mmol) was added drop wise to a solution of cyclohexane-1,3-dione (11.4 g, 102 mmol) in THF (200 ml) at -50° C. The mixture was stirred at -50° C. for 15 min and trifluoromethanesulfonic anhydride (30.1 g, 107 mmol) was added through an addition funnel. After completion of the addition the reaction mixture was allowed to slowly warm to RT. The reaction mixture was then cooled to -30° C., and 200 mL of saturated aqueous sodium bicarbonate was added slowly. The solvent was removed under reduced pressure and the remaining aqueous layer was extracted with EtOAc (2*400 ml). The combined organic layers were washed with brine and dried over sodium sulfate. Filtration and concentration under reduced pressure, followed by flash chromatography on silica gel (0% to 10% EtOAc in hexanes) afforded 3-oxocyclohex-1-enyl trifluoromethanesulfonate as a yellow oil. MS (ESI, pos. ion) m/z: 245.0(M+1).
References:
AMGEN INC. US2010/160280, 2010, A1 Location in patent:Page/Page column 154; 155