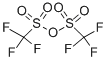
Trifluoromethanesulfonic anhydride synthesis
- Product Name:Trifluoromethanesulfonic anhydride
- CAS Number:358-23-6
- Molecular formula:C2F6O5S2
- Molecular Weight:282.14
A dry, 100-ml., round-bottomed flask is charged with 36.3 g. (0.242 mole) of trifluoromethanesulfonic acid (Note 1) and 27.3 g. (0.192 mole) of phosphorus pentoxide (Note 2). The flask is stoppered and allowed to stand at room temperature for at least 3 hours. During this period the reaction mixture changes from a slurry to a solid mass. The flask is fitted with a short-path distilling head and heated first with a stream of hot air from a heat gun and then with the flame from a small burner.
The flask is heated until no more trifluoromethanesulfonic anhydride
distills, b.p. 82–115°, yielding 28.4–31.2 g. (83–91%) of the anhydride,
a colorless liquid. Although this product is sufficiently pure for use
in the next step, the remaining acid may be removed from the anhydride
by the following procedure. A slurry of 3.2 g. of phosphorus pentoxide
in 31.2 g. of the crude anhydride is stirred at room temperature in a
stoppered flask for 18 hours. After the reaction flask has been fitted
with a short-path distilling head, it is heated with an oil bath,
yielding 0.7 g. of forerun, b.p. 74–81°, followed by 27.9 g. of the pure
trifluoromethanesulfonic acid anhydride, b.p. 81–84° .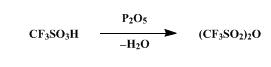
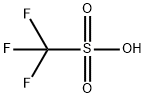
1493-13-6
660 suppliers
$12.00/5g

358-23-6
629 suppliers
$10.00/1g
Yield:358-23-6 96.1%
Reaction Conditions:
with phosphorus pentoxide at 40 - 90;Industrial scale;Concentration;Temperature;
Steps:
1 EXAMPLE 1
EXAMPLE 1 [0059] A kneader-type reactor (actual capacity: 400 L; maximum power: 45 kW) equipped with a jacket and two-shaft blades was charged with 430.0 kg of trifluoromethanesulfonic acid of 99.5 wt% purity, followed by making warm water flow through the jacket to increase temperature of the introduced trifluoromethanesulfonic acid to 40 °C. Then, under a condition that the inside of the reactor was stirred, 180.0 kg of diphosphorus pentoxide powder of 98.5 wt% purity (molar ratio of trifluoromethanesulfonic acid to diphosphorus pentoxide was 2.3) was added. Then, temperature of the warm water flowing through the jacket was increased. To correspond to the second step, while the inside of the reactor was kneaded by a power of 8 kW, the inside temperature of the reactor was increased to 90 °C to generate trifluoromethanesulfonic anhydride, and the inside of the reactor was sucked by using a vacuum pump connected to the reactor to conduct an operation of discharging the generated trifluoromethanesulfonic anhydride from the reactor. The trifluoromethanesulfonic anhydride discharged from the kneader-type reactor was subjected to a liquefaction condensation by cooling at 10 °C using a shell and tube type cooler installed in the middle of piping connecting the reactor with the vacuum pump. The liquefied trifluoromethanesulfonic anhydride was recovered in a product tank. The recovered trifluoromethanesulfonic anhydride was 317.8 kg in weight. As purity of the recovered product was analyzed by using an NMR measurement apparatus (JNM-AL400, made by JEOL), purity was 97.7 wt%. [0060] Then, the warm medium made to flow through the jacket of the kneader-type reactor was changed from warm water to steam, and there was conducted an operation for discharging the unreacted trifluoromethanesulfonic acid from the reactor, which corresponded to the third step, by increasing the inside temperature of the kneader-type reactor until 120 °C and by sucking the inside of the reactor using a vacuum pump connected to the reactor, while kneading the residue of the reactor at a power of 10 kW. The unreacted trifluoromethanesulfonic acid discharged from the reactor was subjected to a liquefaction condensation by cooling to 10 °C using a shell and tube type cooler installed in the middle of the piping connecting the reactor with the vacuum pump. The liquefied trifluoromethanesulfonic acid was recovered in a tank for recovering the unreacted trifluoromethanesulfonic acid. The recovered trifluoromethanesulfonic acid was 85.0 kg in weight. As composition of the recovered product was analyzed by using an NMR measurement apparatus (JNM-AL400, made by JEOL), purity of trifluoromethanesulfonic acid was 98.9 wt%, trifluoromethanesulfonic anhydride was 0.5 wt%, and the trifluoromethanesulfonic acid ester was 0.6 wt%. Yield of trifluoromethanesulfonic anhydride was found to be 96.1 % by conducting a calculation based on trifluoromethanesulfonic acid from which the recovered, unreacted trifluoromethanesulfonic acid was subtracted. 85.0 kg of the recovered, unreacted trifluoromethanesulfonic acid was totally used as the raw material of the next batch.
References:
EP2781506,2014,A1 Location in patent:Paragraph 0059-0061
879545-43-4
1 suppliers
inquiry
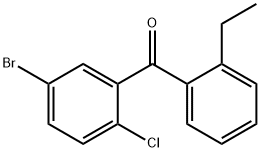
879545-44-5
0 suppliers
inquiry
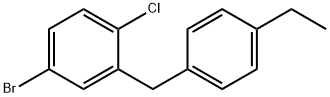
879545-41-2
1 suppliers
inquiry

358-23-6
629 suppliers
$10.00/1g

1493-13-6
660 suppliers
$12.00/5g

358-23-6
629 suppliers
$10.00/1g
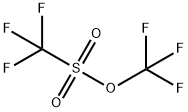
3582-05-6
107 suppliers
$95.00/5G
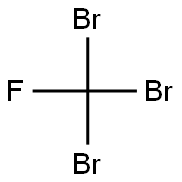
353-54-8
106 suppliers
$51.00/5g
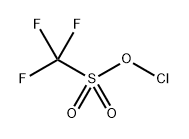
65597-24-2
0 suppliers
inquiry

358-23-6
629 suppliers
$10.00/1g

74501-99-8
0 suppliers
inquiry
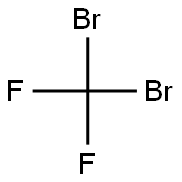
75-61-6
99 suppliers
$95.00/1g

65597-24-2
0 suppliers
inquiry
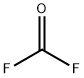
353-50-4
0 suppliers
inquiry

358-23-6
629 suppliers
$10.00/1g
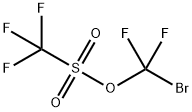
74501-92-1
0 suppliers
inquiry