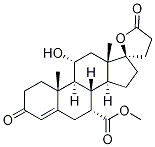
11-a-Hydroxy canrenone methyl ester synthesis
- Product Name:11-a-Hydroxy canrenone methyl ester
- CAS Number:192704-56-6
- Molecular formula:C24H32O6
- Molecular Weight:416.51
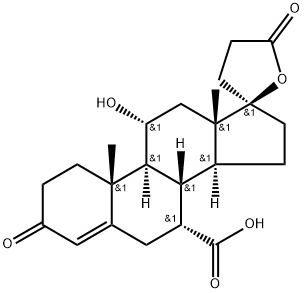
209253-72-5

74-88-4

192704-56-6
Under stirring conditions, 5a (5 g, 12 mmol) was dissolved in DMF (40 ml) and heated to 45 °C. Subsequently, AcOH (2.9 ml, 51 mmol) was added to the solution. after 30 min, NaNO2 (2.484 g, 36 mmol) was added slowly in batches. After 1 hour of reaction, DMF (3 ml) solution of AcOH (2.9 ml, 51 mmol) was added dropwise. After 4 hours of reaction, the mixture was cooled to 0°C and quenched with saturated aqueous NaHCO3 solution. Na2SO3 solution (54 ml, 89 mmol) was added. The mixture was warmed to room temperature and stirred overnight. All solvent was removed under vacuum to give a powdered solid. The solid was diluted with acetone, filtered and the filter cake was washed three times with acetone. The organic layer was combined with the filtrate and concentrated to about 100 ml. To the concentrate was added MeI (1.5 ml, 24 mmol) and K2CO3 (5 g, 36 mmol) at 0 °C. The mixture was warmed to room temperature and stirred overnight. The reaction mixture was filtered and the filter cake was washed three times with DCM. The organic layer was combined with the filtrate and all solvents were removed under vacuum. The residue was diluted with DCM, washed sequentially with 10% aqueous HCl, aqueous NaHCO3 and brine, dried with Na2SO4, filtered and the filtrate was concentrated to give the crude product 6. The crude product can be purified by recrystallization from DCM/toluene to give colorless crystals 6 (4 g, 80% yield). The product characterization data were as follows: melting point 230-232 °C; 1H NMR (300 MHz, CDCl3) δ 5.68 (s, 1H), 4.04 (dt, J = 10.23, 9.84, 3.98 Hz, 1H), 3.64 (s, 3H), 2.85-2.71 (m, 2H), 2.68-2.18 (m, 8H), 2.10 -1.57 (m, 9H), 1.49-1.40 (m, 2H), 1.35 (s, 3H), 1.00 (s, 3H); HR-MS (EI) m/z C24H32O6 (M+) calculated value 416.221, measured value 416.225; Elemental analysis of the calculated value (C24H32O6): C 69.21, H 7.74; measured value C 68.32, H 7.72; UV maximum absorption wavelength 244nm.

209253-72-5
2 suppliers
inquiry

74-88-4
357 suppliers
$15.00/10g

192704-56-6
142 suppliers
$150.00/10mg
Yield:192704-56-6 80%
Reaction Conditions:
with potassium carbonate in acetone at 0 - 20;
Steps:
2.2
2) Cool the crude acetone solution of the compound represented by the above formula 3 to 0°C,After adding iodomethane(1.5ml, 0.024mol) and potassium carbonate (K2CO3) (5g, 0.036mol) and fully stirred, warmed to room temperature and reacted overnight,TLC detects that the raw materials have completely disappeared. Filter to remove K2CO3,Wash the filter cake with dichloromethane (100ml×3) and combine the organic layers,Spin dry. Dissolve with 250ml of dichloromethane,Then use dilute hydrochloric acid (10% w/w), saturated NaHCO3 solution,Wash with saturated sodium thiosulfate solution and saturated brine,Then dry with anhydrous sodium sulfate, filter,Spin-dry to obtain the crude compound of formula 4 and use it directly in the next step.The crude product of the compound represented by Formula 4 was recrystallized with methylene chloride/toluene to obtain colorless transparent crystals (4 g, yield: 80%).
References:
CN103087139,2016,B Location in patent:Paragraph 0078-0079; 0081-0086

209253-72-5
2 suppliers
inquiry

18107-18-1
210 suppliers
$33.00/5g

192704-56-6
142 suppliers
$150.00/10mg
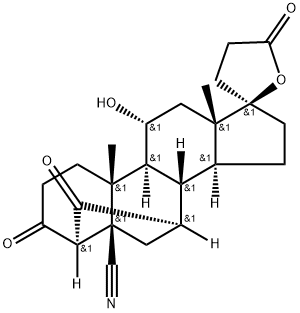
192704-54-4
13 suppliers
inquiry

124-41-4
730 suppliers
$12.00/25g

192704-56-6
142 suppliers
$150.00/10mg

192704-54-4
13 suppliers
inquiry
865-33-8
174 suppliers
$12.50/100G

192704-56-6
142 suppliers
$150.00/10mg
41020-65-9
40 suppliers
inquiry

192704-56-6
142 suppliers
$150.00/10mg