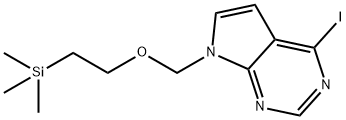
4-iodo-7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidine synthesis
- Product Name:4-iodo-7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidine
- CAS Number:1100319-02-5
- Molecular formula:C12H18IN3OSi
- Molecular Weight:375.28
![7H-Pyrrolo[2,3-d]pyriMidine, 4-iodo-](/CAS/20150408/GIF/1100318-96-4.gif)
1100318-96-4
54 suppliers
$45.00/50mg
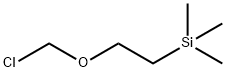
76513-69-4
358 suppliers
$6.00/1g
![4-iodo-7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidine](/CAS/20180601/GIF/1100319-02-5.gif)
1100319-02-5
9 suppliers
$427.00/1g
Yield:1100319-02-5 80%
Reaction Conditions:
Stage #1: 4-iodo-7H-pyrrolo[2,3-d]pyrimidinewith potassium carbonate in N,N-dimethyl-formamide at 20; for 0.5 h;
Stage #2: (2-trimethylethylsilylethoxy)methyl chloride in N,N-dimethyl-formamide at 20; for 6 h;
Steps:
1.2 Step 2: Synthesis of Compound 1C
K2CO3 (2g, 14.6mmol) was added to a solution of 4-iodo-7H-pyrrolo[2,3-d]pyrimidine (1.79g, 7.31mmol) in anhydrous DMF. It was then stirred at room temperature for 30 minutes. After 30 minutes, 2-(trimethylsilyl)ethoxymethyl chloride (1.35 g, 7.31 mmol, 1.44 ml) was added dropwise to the solution. The reaction was stirred at room temperature for 6 hours.TLC showed the reaction was over. The reaction was quenched by the addition of saturated aqueous NH4Cl. Extract with ethyl acetate (3 × 10 mL), combine the organic phases and dry over anhydrous sodium sulfate, filter to remove the desiccant, desolvate under reduced pressure, and purify the residue by silica gel column chromatography (petroleum ether / ethyl acetate = 10: 1 to 3: 1 (volume ratio V: V)) to obtain the target compound 1C (2.2 g, yellow oil) in a yield of 80%.
References:
CN110357887,2019,A Location in patent:Paragraph 0063-0066; 0070-0072
![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/CAS/GIF/3680-69-1.gif)
3680-69-1
837 suppliers
$6.00/1g
![4-iodo-7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidine](/CAS/20180601/GIF/1100319-02-5.gif)
1100319-02-5
9 suppliers
$427.00/1g