
tert-butyl (1R,2S)-3,3-difluoro-2-hydroxycyclohexylcarbamate synthesis
- Product Name:tert-butyl (1R,2S)-3,3-difluoro-2-hydroxycyclohexylcarbamate
- CAS Number:1109284-41-4
- Molecular formula:C11H19F2NO3
- Molecular Weight:251.27

24424-99-5
870 suppliers
$13.50/25G
![Cyclohexanol, 2,2-difluoro-6-[[(1R)-1-phenylethyl]amino]-, (1S,6R)-](/CAS/20200401/GIF/1109284-39-0.gif)
1109284-39-0
3 suppliers
inquiry

1109284-41-4
11 suppliers
inquiry
Yield:1109284-41-4 67%
Reaction Conditions:
with palladium hydroxide 10 wt. % on activated carbon;palladium on activated carbon;hydrogen in methanol at 50; under 2585.81 Torr; for 16 h;
Steps:
4 Step 4 - Tert-butyl N-[(1R,2S)-3,3-difluoro-2-hydroxy-cyclohexyl]carbamate
A mixture of (1S,6R)-2,2-difluoro-6-[[(1R)-1-phenylethyl]amino]cyclohexanol (1.50 g, 5.88 mmol), Boc2O (1.41 g, 6.46 mmol), Pd(OH)2/C (300 mg, 5.88 mmol, 10% wt) and Pd/C (300 mg, 10% wt) in methanol (30 mL) was stirred at 50 °C under hydrogen (50 psi) for 16 hours. On completion, the mixture was filtered and the cake was washed with methanol (20 mL). The filtrate and washing were combined and concentrated in vacuo. The residue was purified by column chromatography on silica gel (PE: EA = 20:1 to 5:1) to give the title compound (1.00 g, 67% yield) as white solid.1H NMR (400 MHz, CDCl3) d 4.72 - 4.58 (m, 1H), 3.76 - 3.63 (m, 1H), 3.61 - 3.47 (m, 1H), 3.11 (m, 1H), 2.24 - 2.12 (m, 1H), 2.08 - 1.99 (m, 1H), 1.80 - 1.59 (m, 3H), 1.46 (s, 9H), 1.40 - 1.30 (m, 1H).
References:
WO2020/113233,2020,A1 Location in patent:Paragraph 002998; 003005-003006

24424-99-5
870 suppliers
$13.50/25G
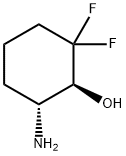
1109284-40-3
24 suppliers
inquiry

1109284-41-4
11 suppliers
inquiry
![Cyclohexanol, 2,2-difluoro-6-[[(1R)-1-phenylethyl]amino]-, (1S,6R)-](/CAS/20200401/GIF/1109284-39-0.gif)
1109284-39-0
3 suppliers
inquiry

1109284-41-4
11 suppliers
inquiry
![7-OXABICYCLO[4.1.0]HEPTAN-2-ONE](/CAS/GIF/6705-49-3.gif)
6705-49-3
80 suppliers
$18.00/250mg

1109284-41-4
11 suppliers
inquiry
![2,2-DIFLUORO-7-OXA-BICYCLO[4.1.0]HEPTANE](/CAS/GIF/1109284-38-9.gif)
1109284-38-9
28 suppliers
$65.00/50mg

1109284-41-4
11 suppliers
inquiry