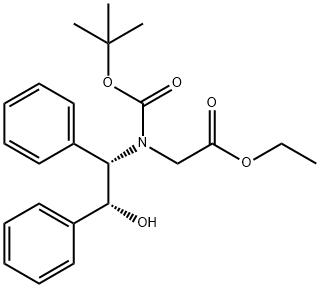
N-(tert-Butyloxycarbonyl)-N-[(1S,2R)-2-hydroxy-1,2-diphenylethyl]-glycine Ethyl Ester synthesis
- Product Name:N-(tert-Butyloxycarbonyl)-N-[(1S,2R)-2-hydroxy-1,2-diphenylethyl]-glycine Ethyl Ester
- CAS Number:112741-70-5
- Molecular formula:C23H29NO5
- Molecular Weight:399.48

24424-99-5
862 suppliers
$13.50/25G
![N-[(1S,2R)-2-Hydroxy-1,2-diphenylethyl]-glycine Ethyl Ester](/CAS/GIF/100678-82-8.gif)
100678-82-8
32 suppliers
$503.13/5MG
![N-(tert-Butyloxycarbonyl)-N-[(1S,2R)-2-hydroxy-1,2-diphenylethyl]-glycine Ethyl Ester](/CAS/GIF/112741-70-5.gif)
112741-70-5
15 suppliers
$498.38/5MG
Yield:112741-70-5 85%
Reaction Conditions:
with sodium hydrogencarbonate in chloroform;water at 70; for 24 h;
Steps:
1.3
A solution of (1.8 g, 6.0 mmol) of 2 - ((1S, 2R) -2-hydroxy-1,2-diphenylethylamino)-acetic acid ethyl ester in 20 ml of CHCl 3 was added 20 ml of saturated aqueous NaHCO 3 solution, g (Boc) 2O anhydride added to the reaction flask, heated to 70 ° C reflux, refluxed to room temperature after 24 hours. The reaction mixture was transferred to a separatory funnel, the organic phase was separated and the aqueous phase was extracted with CHCl3. The organic phases were combined, dried, filtered and concentrated to give 2.1 g of N-Boc substituted diphenylamino alcohol with a yield of 85% .
References:
CN104945345,2017,B Location in patent:Paragraph 0105; 0107; 0116; 0117
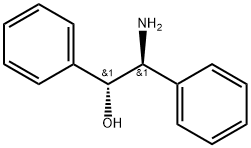
23190-16-1
280 suppliers
$10.00/1g
![N-(tert-Butyloxycarbonyl)-N-[(1S,2R)-2-hydroxy-1,2-diphenylethyl]-glycine Ethyl Ester](/CAS/GIF/112741-70-5.gif)
112741-70-5
15 suppliers
$498.38/5MG
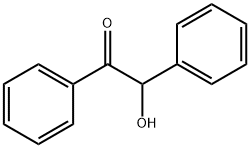
119-53-9
402 suppliers
$5.00/25g
![N-(tert-Butyloxycarbonyl)-N-[(1S,2R)-2-hydroxy-1,2-diphenylethyl]-glycine Ethyl Ester](/CAS/GIF/112741-70-5.gif)
112741-70-5
15 suppliers
$498.38/5MG
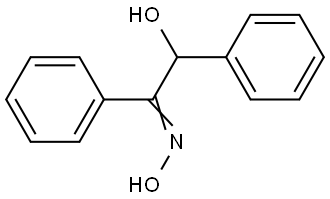
441-38-3
195 suppliers
$6.00/5g
![N-(tert-Butyloxycarbonyl)-N-[(1S,2R)-2-hydroxy-1,2-diphenylethyl]-glycine Ethyl Ester](/CAS/GIF/112741-70-5.gif)
112741-70-5
15 suppliers
$498.38/5MG