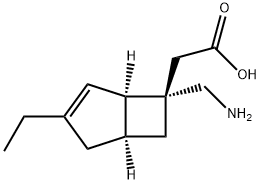
Mirogabalin synthesis
- Product Name:Mirogabalin
- CAS Number:1138245-13-2
- Molecular formula:C12H19NO2
- Molecular Weight:209.28
![tert-butyl 2-((1R,5S,6S)-6-(((tert-butoxycarbonyl)amino)methyl)-3-ethylbicyclo[3.2.0]hept-3-en-6-yl)acetate](/CAS/20180601/GIF/1138246-20-4.gif)
1138246-20-4

1138245-13-2
1. (1R,5S,6S)-[6-(tert-butoxycarbonylaminomethyl)-3-ethylbicyclo[3.2.0]hept-3-en-6-yl]acetate (9.82 g, 23.7 mmol) was added to 100 mL of 4N hydrochloric acid-ethyl acetate solution. 2. The reaction mixture was stirred at room temperature for 1 hour. 3. After completion of the reaction, the solvent was removed by distillation under reduced pressure. 4. The residue was dissolved in dichloromethane. 5. 5. Triethylamine was added dropwise to the above solution and the resulting white powder was subsequently collected by filtration. 6. The collected powder was washed with dichloromethane and then dried to give 4.02 g of white powder. 7. Finally, the powder was washed with ethanol and ethyl acetate to afford the target compound 2-((1R,5S,6S)-6-(aminomethyl)-3-ethylbicyclo[3.2.0]hept-3-en-6-yl)acetic acid as a white powder (2.14 g, 43% yield).
![tert-butyl 2-((1R,5S,6S)-6-(((tert-butoxycarbonyl)amino)methyl)-3-ethylbicyclo[3.2.0]hept-3-en-6-yl)acetate](/CAS/20180601/GIF/1138246-20-4.gif)
1138246-20-4
4 suppliers
inquiry

1138245-13-2
192 suppliers
$55.00/1mg
Yield:1138245-13-2 43%
Reaction Conditions:
Stage #1:tert-butyl [(1R,5S,6S)-6-(tert-butoxycarbonylamino)methyl-3-ethylbicyclo[3.2.0]hept-3-en-6-yl]acetate with hydrogenchloride in water;ethyl acetate at 20; for 1 h;
Stage #2: with triethylamine in dichloromethane
Steps:
21.c (21-c)
(21-c) [(1R,5S,6S)-6-aminomethyl-3-ethylbicyclo[3.2.0]hept-3-en-6-yl]acetic acid A 4 N hydrochloric acid-ethyl acetate solution (100 mL) was added to tert-butyl (1R,5S,6S)-[6-(tert-butoxycarbonylaminomethyl)-3-ethylbicyclo[3.2.0]hept-3-en-6-yl]acetate (9.82 g, 23.7 mmol), and the mixture was stirred at room temperature for 1 hour. Then, the solvent was distilled off under reduced pressure. The residue was dissolved in dichloromethane. To the solution, triethylamine was added dropwise, and the resulting powder was collected by filtration, then washed with dichloromethane, and then dried to obtain 4.02 g of a white powder. This powder was washed with ethanol and ethyl acetate to obtain the title compound of interest as a white powder (2.14 g, 43%).
References:
Daiichi Sankyo Company, Limited EP2192109, 2010, A1 Location in patent:Page/Page column 45