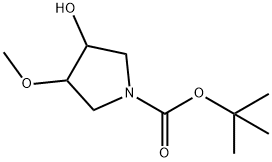
tert-butyl 3-hydroxy-4-methoxypyrrolidine-1-carboxylate synthesis
- Product Name:tert-butyl 3-hydroxy-4-methoxypyrrolidine-1-carboxylate
- CAS Number:114214-50-5
- Molecular formula:C10H19NO4
- Molecular Weight:217.26

124-41-4
730 suppliers
$12.00/25g
![3-Boc-6-oxa-3-aza-bicyclo[3.1.0]hexane](/CAS/GIF/114214-49-2.gif)
114214-49-2
173 suppliers
$9.00/1g

114214-50-5
5 suppliers
inquiry
Yield:114214-50-5 65%
Reaction Conditions:
in methanol at 60; for 5 h;
Steps:
6.1 The first step (3R,4R)-3-hydroxy-4-methoxypyrrolidine-1-carboxylate tert-butyl ester 6b-1&(3S,4S)-3-hydroxy-4-methoxypyrrolidine-1- tert-Butyl formate 6b-2
3-N-tert-butoxycarbonyl-6-oxa-3-azabicyclo[3.1.0]hexane 6a (10.6 g, 57.2 mmol) was dissolved in methanol (50 mL), and sodium methoxide ( 3.71 g, 68.7 mmol), the temperature was raised to 60° C. for 5 hours, and the reaction of the raw materials was monitored by TLC for completeness.The reaction solution was cooled to room temperature, diluted with water, extracted with ethyl acetate, the organic phases were combined, washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and the crude product was purified by silica gel column chromatography to obtain the title compound 6b (8.0 g, yield) as a white solid. 65%).Compound 6b (6.0g) was subjected to chiral resolution (LC016-1, 21.2*250mm, 5um, 20mL/min, EtOH:Hexane=5:95) to obtain compound 6b-2 (peak No. 1, RT 10.62min) (1.8g , yield 77%) and compound 6b-1 (peak No. 2, RT 13.01 min) (2.3 g, yield 60%).
References:
WO2022/170947,2022,A1 Location in patent:Page/Page column 23-24
![3-Boc-6-oxa-3-aza-bicyclo[3.1.0]hexane](/CAS/GIF/114214-49-2.gif)
114214-49-2
173 suppliers
$9.00/1g

114214-50-5
5 suppliers
inquiry