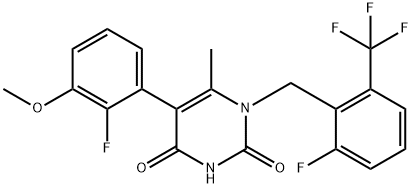
5-(2-Fluoro-3-methoxyphenyl)-1-[[2-fluoro-6-(trifluoromethyl)phenyl]methyl]-6-methyl-2,4(1H,3H)-pyrimidinedione synthesis
- Product Name:5-(2-Fluoro-3-methoxyphenyl)-1-[[2-fluoro-6-(trifluoromethyl)phenyl]methyl]-6-methyl-2,4(1H,3H)-pyrimidinedione
- CAS Number:1150560-59-0
- Molecular formula:C20H15F5N2O3
- Molecular Weight:426.34
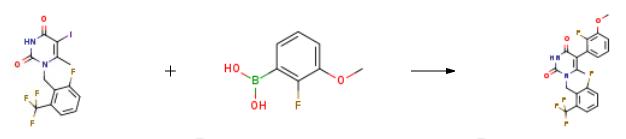
To a reactor was charged l-(2-fluoro-6-trifluoromethyl-benzyl)-5-iodo-6-methyl- lH-pyrimidine-2,4-dione Ib (5.0 kg), 2-fluoro-3-methoxyphenylboronic acid (2.58 kg), and acetone (5.5 L). The mixture was agitated and a potassium hydroxide/water solution (2.658 kg/19.0 L) was charged. The reactor contents were degassed for 30-60 min, then the internal temperature was adjusted to 40 0C. l,l-(bis-di-t-butylphosphino)ferrocene palladium dichloride (11.4 g) was added to the reactor and the contents mixed with jacket temperature set to 45 0C until the reaction was complete (2.5 hr). The reaction mixture was cooled to 20-30 0C. Celite (1.25 kg) was charged to the reactor and stirred for more than one hour and the mixture was filtered through a Celite pad (0.51 kg). The reactor and Celite cake were washed with acetone/water/KOH (2.6 L/7.5 L/0.38 kg). The filtered solutions were passed through a line filter and added over a period of 1-1.5 hr to a mixture of THF/AcOH/Water (15.0 L/7.53 L/5.0 L) maintained at 62 0C. The reactor contents were cooled to 20 0C over 2-3 hr. The mixture was filtered and the cake washed with 60:40 water/MeOH (2 x 12.6 L) followed by methanol (2 x 16 L). The solid was dried in a vacuum oven at 65 0C for 18 hr to provide 5-(2-fluoro-3-methoxy- phenyl)-l-(2-fluoro-6-trifluoromethyl-benzyl)-6-methyl-lH-pyrimidine-2,4-dione Ic (4.312 kg, 87 % molar yield) as an off-white solid. LCMS (ESI) m/z 427.1 (MH+). If necessary, the material may be solubilized, re-treated with Celite, and crystallized as above to increase purity.
830346-48-0
148 suppliers
$13.00/250mg
352303-67-4
366 suppliers
$5.00/1g
![5-(2-Fluoro-3-methoxyphenyl)-1-[[2-fluoro-6-(trifluoromethyl)phenyl]methyl]-6-methyl-2,4(1H,3H)-pyrimidinedione](/CAS/20150408/GIF/1150560-59-0.gif)
1150560-59-0
156 suppliers
inquiry
Yield:1150560-59-0 79.2%
Reaction Conditions:
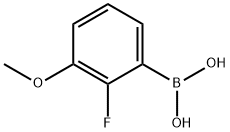
Stage #1:(2-fluoro-3-methoxyphenyl)boronic acid with potassium iodide;potassium hydroxide in water;acetone at 25 - 30; for 0.166667 h;Inert atmosphere;
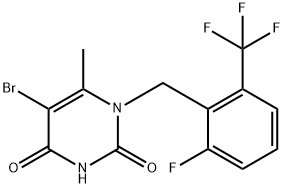
Stage #2:5-bromo-1-[2-fluoro-6-(trifluoromethyl)benzyl]-6-methylpyrimidine-2,4-(1H,3H)-dione with tri tert-butylphosphoniumtetrafluoroborate in water;acetone at 25 - 45; for 0.583333 h;Inert atmosphere;Further stages;
Steps:
2.1; 3.1 Stage-1: Synthesis of 5-(2-fluoro-3-methoxyphenyl)-1-(2-fluoro-6-(trifluoromethyl) benzyl)- 6-methylpyrimidine-2,4(1H,3H)-dione [compound of formula-IV]
Aqueous potassium hydroxide solution is added to a mixture of (2-fluoro-3-methoxyphenyl) boronic acid [compound of formula-III] (199.8 grams), potassium iodide (65.3 grams), acetone (900 mL) and water (900 mL) at 25-30 °C under nitrogen atmosphere and stirred for 10 minutes at the same temperature. 1 -(2-fluoro-6-(trifluoromethyl) benzyl)-5-bromo-6-methyl-1H- pyrimidine-2,4-dione [Compound of formula-II] (150 grams) and tri tert-butyl phosphonium tetrafluoroborate (1.14 grams) were added to the resulting reaction mixture at 25-30 °C and stirred for 15 minutes at the same temperature. Heated the reaction mixture to 40-45 °C and stirred for 20 minutes at the same temperature, palladium acetate (2.94 grams) was added to the resulting reaction mixture at 40-45°C. Heated the reaction mixture to 65-75 °C and stirred for 8 hours at the same temperature. Acetic acid was slowly added drop wise to the reaction mixture at 60-65 °C and stirred for 30 minutes at the same temperature. Cooled the reaction mixture to 25-30 °C and stirred for 2 hours at the same temperature. Filtered the reaction mixture and washed with water followed by methanol. Dissolve the obtained wet material in methanol at 25-30°C and stirred for 30 minutes at the same temperature. Filtered the precipitated solid, washed with water followed by methanol and dried to get the title compound.Yield: 79.2 %
References:
NEULAND LABORATORIES LIMITED WO2021/64561, 2021, A1 Location in patent:Page/Page column 13; 18; 19-20
![1-[2-fluoro-6-(trifluoromethyl)benzyl]-5-iodo-6-methylpyrimidine-2,4(1H,3H)-dione](/CAS/GIF/1150560-54-5.gif)
1150560-54-5
156 suppliers
inquiry

352303-67-4
366 suppliers
$5.00/1g
![5-(2-Fluoro-3-methoxyphenyl)-1-[[2-fluoro-6-(trifluoromethyl)phenyl]methyl]-6-methyl-2,4(1H,3H)-pyrimidinedione](/CAS/20150408/GIF/1150560-59-0.gif)
1150560-59-0
156 suppliers
inquiry