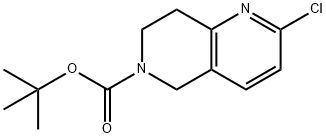
tert-butyl 2-chloro-7,8-dihydro-1,6-naphthyridine-6(5H)-carboxylate synthesis
- Product Name:tert-butyl 2-chloro-7,8-dihydro-1,6-naphthyridine-6(5H)-carboxylate
- CAS Number:1151665-15-4
- Molecular formula:C13H17ClN2O2
- Molecular Weight:268.74
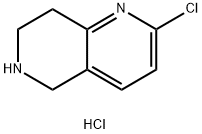
766545-20-4

24424-99-5

1151665-15-4
A slurry was formed in dichloromethane (DCM, 1 L) using 2-chloro-5,6,7,8-tetrahydro-1,6-naphthyridine hydrochloride (106.1 g, 517 mmol) and N,N-diisopropylethylamine (80 g, 108 mL, 621 mmol, 1.2 equiv.) as starting materials. A solution of di-tert-butyl dicarbonate (119 g, 543 mmol, 1.05 eq.) in DCM (100 mL) was slowly added through the addition funnel over a period of 1 hour. The reaction mixture was gradually converted to a clarified solution, followed by continued stirring at room temperature for 1 h. The reaction progress was monitored by LCMS. Upon completion of the reaction, the mixture was concentrated. The concentrated residue was dissolved in ethyl acetate (EtOAc, 1 L), washed sequentially with water (3 x 300 mL) and brine (300 mL), and the organic phase was dried with anhydrous magnesium sulfate (MgSO4). The solvent was removed by evaporation under reduced pressure to afford 2-chloro-7,8-dihydro-1,6-naphthyridine-6(5H)-carboxylic acid 1,1-dimethylethyl ester as an off-white solid (139 g, yield: 100%). The product was characterized by 1H NMR (400 MHz, CDCl3) and LCMS: 1H NMR δ 1.49 (9H, s), 2.97 (2H, t, J = 5.9 Hz), 3.73 (2H, t, J = 6.0 Hz), 4.57 (2H, s), 7.17 (1H, d, J = 8.0 Hz), 7.38 (1H, d, J = 8.0 Hz) ppm; LCMS m/z: 269 (M + 1).

766545-20-4
133 suppliers
$50.00/1g

24424-99-5
868 suppliers
$13.50/25G

1151665-15-4
94 suppliers
$45.00/100mg
Yield: 100%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at 20; for 2 h;
Steps:
1
To a slurry of 2-chloro-5,6,7,8-tetrahydro-1,6-naphthyridine hydrochloride (106.1 g, 517 mmol, commercially available from D-L Chiral Chemicals, ST-0143) and N,N-diisopropylethylamine (80 g, 108 mL, 621 mmol, 1.2 eq) in DCM (1 L) was added a solution of di-tert-butyl dicarbonate (119 g, 543 mmol, 1.05 eq) in DCM (100 mL) via an addition funnel within 1 hr. The reaction mixture became a clean solution and the solution thus obtained was stirred at room temperature for an additional hour and monitored using LCMS. Upon completion, the reaction mixture was concentrated. The residue was dissolved in EtOAc (1 L) and washed with water (3*300 mL), washed with brine (300 mL) and dried over Mg504. The solvent was evaporated under vacuum to give the title compound as an off-white solid (139 g, yield: 100%). 1H NMR (400 MHz, CDCl3) δ ppm 1.49 (9H, s), 2.97 (2H, t, J=5.9 Hz), 3.73 (2H, t, J=6.0 Hz), 4.57 (2H, s), 7.17 (1H, d, J=8.0 Hz), 7.38 (1H, d, J=8.0 Hz) ppm; LCMS m/z: 269 (M+1).
References:
Amgen Inc. US2012/244110, 2012, A1 Location in patent:Page/Page column 61
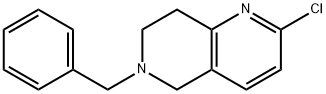
210539-04-1
85 suppliers
$276.00/250mg

24424-99-5
868 suppliers
$13.50/25G

1151665-15-4
94 suppliers
$45.00/100mg