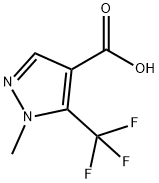
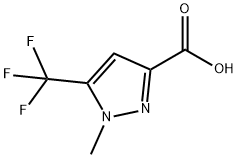
1-METHYL-5-(TRIFLUOROMETHYL)-1H-PYRAZOLE-4-CARBOXYLIC ACID synthesis
- Product Name:1-METHYL-5-(TRIFLUOROMETHYL)-1H-PYRAZOLE-4-CARBOXYLIC ACID
- CAS Number:119083-00-0
- Molecular formula:C6H5F3N2O2
- Molecular Weight:194.11
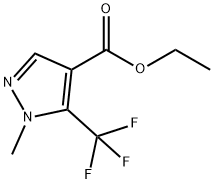
231285-86-2

119083-00-0
The general procedure for the synthesis of 1-methyl-5-(trifluoromethyl)-1H-pyrazole-4-carboxylic acid from ethyl 1-methyl-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate is as follows: Preparation of intermediate 65b: Ethyl 1-methyl-5-(trifluoromethyl)pyrazole-4-carboxylate (2.91 g, 13.0 mmol) was dissolved in THF (52 mL) and cooled to 0 °C. Subsequently, a solution of lithium hydroxide (627 mg, 26.1 mmol) in water (26 mL) was slowly added. The reaction mixture was stirred overnight at room temperature. After completion of the reaction, the mixture was acidified to pH 1-2 with 1 M HCl and extracted with EtOAc (4 x 50 mL). The organic layers were combined, washed with brine (50 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to afford 1-methyl-5-(trifluoromethyl)pyrazole-4-carboxylic acid (2.39 g, 12.33 mmol, 94% yield) as an off-white solid. The product characterization data are as follows: 1H NMR (CDCl3, 400 MHz) δ/ppm: 8.02 (1H, s), 4.13 (3H, q, J = 2.0 Hz) Exchangeable protons. 19F NMR (CDCl3, 400 MHz) δ/ppm: -57.2. MS Method 2: RT: 1.16 min, m/z 193.0 [M-H]-.

231285-86-2
64 suppliers
$24.00/100mg

119083-00-0
72 suppliers
$62.00/250mg
Yield: 94%
Reaction Conditions:
with water;lithium hydroxide in tetrahydrofuran at 0 - 20;
Steps:
Intermediate 65b: I -methyl-5-(trifluoromethyl)pyrazole-4-carboxylic acid
[00618] Intermediate 65b: I -methyl-5-(trifluoromethyl)pyrazole-4-carboxylic acid[00619] To a solution of ethyl 1 -methyl-5-(trifluoromethyl)pyrazole-4-carboxylate (2.91 g, 13.Ogmmol) in THF (52mL) at 0 °Cwas added a solution of lithium hydroxide (627mg, 26.l8mmol) in water (26mL) and the mixture was stirred at room temperature overnight. The mixture was thenacidified to pH 1-2 with 1M HCI and extracted with EtOAc (4 x 5OmL). The combined organic layers were washed with brine (5OmL), dried over Na2SO4, filtered and concentrated in vacuo to give 1- methyl-5-(trifluoromethyl)pyrazole-4-carboxylic acid (2.39g, 12.33mmol, 94% yield) as an off-white solid.1H NMR (CDCI3, 400MHz) O/ppm: 8.02 (1H, 5), 4.13 (3H, q, J= 2.0Hz) exchangeable proton notseen. 19F NMR (CDCI3, 400MHz) O/ppm: -57.2.MS Method 2: RT: 1.16 mi mlz 193.0 [M-H]
References:
REDX PHARMA PLC;ARMER, Richard;BELFIELD, Andrew;BINGHAM, Matilda;JOHNSON, Alice;MARGATHE, Jean-Francois;AVERY, Craig;HUGHES, Shaun;MORRISON, Angus WO2016/51193, 2016, A1 Location in patent:Paragraph 00615; 00618; 00619

124-38-9
134 suppliers
$175.00/23402
497832-98-1
51 suppliers
$45.00/50mg

119083-00-0
72 suppliers
$62.00/250mg

231285-86-2
64 suppliers
$24.00/100mg
111493-74-4
118 suppliers
$16.00/1g
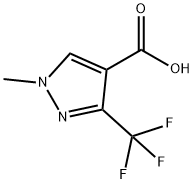
113100-53-1
218 suppliers
$7.00/100mg

119083-00-0
72 suppliers
$62.00/250mg

124-38-9
134 suppliers
$175.00/23402
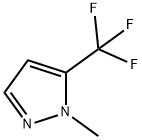
153085-15-5
28 suppliers
inquiry
481065-92-3
50 suppliers
$70.00/250mg

119083-00-0
72 suppliers
$62.00/250mg

153085-15-5
28 suppliers
inquiry

119083-00-0
72 suppliers
$62.00/250mg