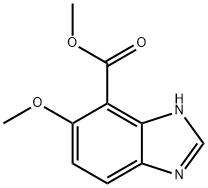
methyl 5-methoxy-3H-benzo[d]imidazole-4-carboxylate synthesis
- Product Name:methyl 5-methoxy-3H-benzo[d]imidazole-4-carboxylate
- CAS Number:1193789-02-4
- Molecular formula:C10H10N2O3
- Molecular Weight:206.2
Yield:-
Reaction Conditions:
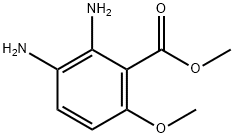
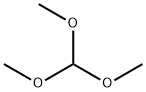
Stage #1: methyl 2,3-diamino-6-methoxybenzoate;trimethyl orthoformate in methanol;
Stage #2: with hydrogenchloride in 1,4-dioxane;methanol at 20; for 0.5 h;Product distribution / selectivity;
Steps:
18.a
To a solution of methyl 2-amino-6-(methyloxy)-3-nitrobenzoate (prepared as in Example Ib) (0.452 g, 1.998 mmol) in ethyl acetate (10.0 mL) was added 10% palladium on charcoal (0.106 g, 0.100 mmol) followed by evacuation of the reaction vessel and purging with 1 atmosphere of hydrogen. Following stirring at ambient temperature for 4 h, the reaction mixture was filtered through Celite, washed through with ethyl acetate, and concentrated in vacuo. The resulting residue was dissolved in methanol (5.0 mL) and treated with trimethyl orthoformate (2.20 mL, 19.90 mmol) followed by hydrochloric acid (4.0 M solution in 1,4-dioxane) (1.00 mL, 4.00 mmol). After stirring 30 min. at ambient temperature, the reaction mixture was neutralized with saturated aqueous sodium bicarbonate, diluted with brine, and extracted thrice with ethyl acetate. The combined organic portions were dried over MgSO/t, filtered, concentrated in vacuo, and triturated with 1 : 1 :1 methanol/diethyl ether/hexanes to afford the title compound (0.347 g, 84%) as a pale orange solid. 1H NMR (400 MHz, OMSO-d6) δ ppm 12.2 (br. s., 1 H), 8.13 (s, 1 H), 7.84 (d, J=9.1 Hz, 1 H), 7.05 (d, J=9.1 Hz, 1 H), 3.87 (s, 3 H), 3.87 (s, 3 H). MS(ES+) m/e 207 [M+H]+.
References:
WO2009/134850,2009,A1 Location in patent:Page/Page column 33