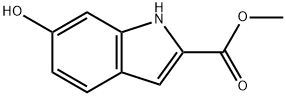
METHYL 6-HYDROXY-1H-INDOLE-2-CARBOXYLATE synthesis
- Product Name:METHYL 6-HYDROXY-1H-INDOLE-2-CARBOXYLATE
- CAS Number:116350-38-0
- Molecular formula:C10H9NO3
- Molecular Weight:191.18
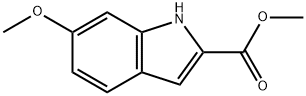
98081-83-5
141 suppliers
$24.00/1g

116350-38-0
67 suppliers
$45.00/25mg
Yield:116350-38-0 85%
Reaction Conditions:
Stage #1:methyl 6-methoxy-1H-indole-2-carboxylate with boron tribromide in dichloromethane at -78 - 0; for 0.833333 h;
Stage #2: with water in dichloromethane at 0;
Steps:
73.1.1
To a dichloromethane (10 mL) solution of 6-methoxy-1H-indole-2-carboxylic acid methyl ester (500 mg, 2.44 mmol) was added boron tribromide (12.2 mL, 12.2 mmol) at -78°C, which was stirred for 50 minutes at 0°C under nitrogen atmosphere. To the reaction solution was added water at 0°C followed by extraction with ethyl acetate. The organic layer was washed with sat. NaCl followed by drying over anhydrous magnesium sulfate and filtering. The filtrate was concentrated under a reduced pressure and the residue was purified by silica gel column chromatography (heptane:ethyl acetate = 1:1) to obtain the title compound (395 mg, 85%) as a white solid. 1H-NMR spectrum (CDCl3) δ (ppm): 3.92 (3H, s), 4.97 (1H, s), 6.74 (1H, dd, J=2.2 Hz, 8.6 Hz), 6.82 (1 H, s), 7.16 (1 H, dd, J=1.1 Hz, 2.2 Hz), 7.54 (1 H, d, J=8.8 Hz), 8.70 (1 H, brs).
References:
Eisai R&D Management Co., Ltd. EP1864980, 2007, A1 Location in patent:Page/Page column 51
103781-89-1
44 suppliers
$60.00/0.25 / G

116350-38-0
67 suppliers
$45.00/25mg
40047-23-2
90 suppliers
$30.00/250mg

18107-18-1
210 suppliers
$33.00/5g

116350-38-0
67 suppliers
$45.00/25mg

67-56-1
790 suppliers
$7.29/5ml-f

40047-23-2
90 suppliers
$30.00/250mg

116350-38-0
67 suppliers
$45.00/25mg
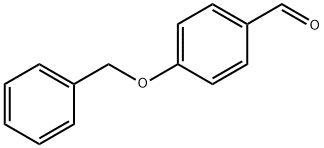
4397-53-9
335 suppliers
$8.00/10g

116350-38-0
67 suppliers
$45.00/25mg