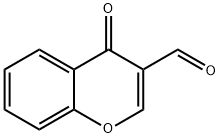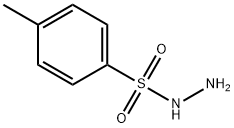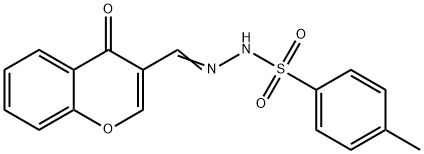
Benzenesulfonic acid, 4-methyl-, 2-[(4-oxo-4H-1-benzopyran-3-yl)methylene]hydrazide synthesis
- Product Name:Benzenesulfonic acid, 4-methyl-, 2-[(4-oxo-4H-1-benzopyran-3-yl)methylene]hydrazide
- CAS Number:120757-41-7
- Molecular formula:C17H14N2O4S
- Molecular Weight:342.37
Yield:120757-41-7 68%
Reaction Conditions:
in ethanol;Reflux;
Steps:
2.1.1. General Procedure for the Synthesis of Sulphonylhydrazones(3a-i)
General procedure: All the sulphonylhydrazones (3a-i) were synthesized, asillustrated in Scheme 1. First of all, 4-methylbenzenesulfonohydrazide(0.01 mol) was taken and dissolved inwarm ethanol. Then equimolar (un)substituted 3-formylchromone was taken and dissolved in a minimumamount of ethanol. After that, both solutions were mixed in around bottom flask and mixture was allowed to reflux for 3-4h. The progress of the reaction was observed from time totime by thin-layer chromatography using ethyl acetate and nhexane(1:1) as the mobile phase. After completion of thereaction, the reaction mixture was left overnight. The nextday, the solid was filtered and recrystallized from acetonitrileand ethanol. The compounds were obtained in good yield,which is presented in Table 1.
References:
Iqbal, Jamshed;Basharat, Ayesha;Bano, Sehrish;Abid, Syed Mobasher Ali;Pelletier, Julie;Sévigny, Jean [Medicinal Chemistry,2021,vol. 17,# 8,p. 866 - 874]