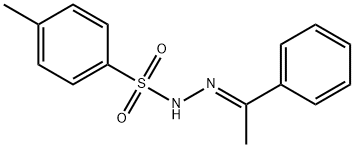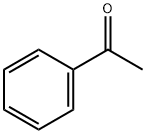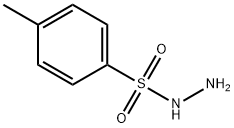
4-Methylbenzenesulfonhydrazide synthesis
- Product Name:4-Methylbenzenesulfonhydrazide
- CAS Number:1576-35-8
- Molecular formula:C7H10N2O2S
- Molecular Weight:186.23
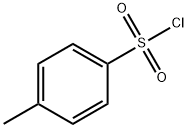
98-59-9

1576-35-8
General procedure for the synthesis of p-toluenesulfonyl hydrazide from p-toluenesulfonyl chloride: general method: preparation of p-toluenesulfonyl hydrazide. A mixed solution of water (300 mL) and hydrazine hydrate (12.5 g; 0.25 mol) was cooled to about 5 °C in an ice-water bath. The reaction temperature was kept below 8 °C while a tetrahydrofuran (50 mL) solution of p-toluenesulfonyl chloride (5.54 g, 0.025 mol) was slowly added under stirring. After the addition was completed, the reaction mixture was continued to be stirred at room temperature for 30 min. Subsequently, the tetrahydrofuran was removed by distillation under reduced pressure. The white solid product was isolated by filtration and washed with cold water (3 x 15 mL). Finally, the product was dried in air overnight to give pure p-toluenesulfonyl hydrazide (4.86 g; 89% yield).
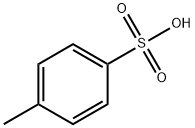
104-15-4
764 suppliers
$133.00/500g

1576-35-8
468 suppliers
$10.00/1g
Yield:1576-35-8 94%
Reaction Conditions:
with carbazic acid in neat (no solvent) at 100; for 5 h;Green chemistry;
Steps:
17
General procedure: 7.6 g (100.0 mmol) of solid hydrazine (H3N + NHCO2-) and adipic acid (7.3 g, 50.0 mmol) were mixed in a mortar without a solvent for 10 minutes and stirred at 100 ° C for 5 hours. To confirm the structure and composition of the product produced in this process and analyzed using 400 MHz NMR (nuclear magnetic resonance) and elemental analysis) .As a result of the analysis, the product was adipohydrazide (C6H14N4O2), the conversion rate was 96% or more, and the yield was 95% or more. Yield (8.27 g, 96% or more); The product was obtained in the same manner as in Example 1 except that 17.21 g of 4-methylbenzenesulfonic acid was used instead of adipic acid. As a result of the analysis, the product was 4-methylbenzenesulfono-hydrazide, the selectivity was 96% or more, and the yield was 94%. Yield: (17.48 g, 94%);
References:
KR2015/88523,2015,A Location in patent:Paragraph 0128; 0212-0214

98-59-9
624 suppliers
$9.00/5g

1576-35-8
468 suppliers
$10.00/1g
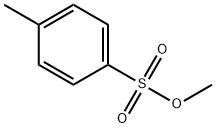
80-48-8
459 suppliers
$5.00/25g

1576-35-8
468 suppliers
$10.00/1g
![1-[(4-Methylphenyl)sulfonyl]-1H-imidazole](/CAS/GIF/2232-08-8.gif)
2232-08-8
215 suppliers
$6.00/5g

1576-35-8
468 suppliers
$10.00/1g