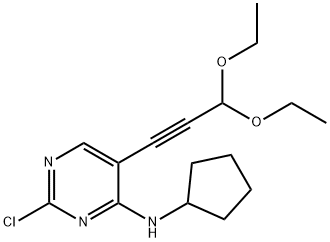
2-chloro-N-cyclopentyl-5-(3,3-diethoxy-1-propyn-1-yl)-4-Pyrimidinamine synthesis
- Product Name:2-chloro-N-cyclopentyl-5-(3,3-diethoxy-1-propyn-1-yl)-4-Pyrimidinamine
- CAS Number:1211442-87-3
- Molecular formula:C16H22ClN3O2
- Molecular Weight:323.82
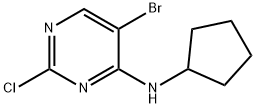
733039-20-8
279 suppliers
$6.00/1g
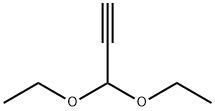
10160-87-9
224 suppliers
$8.00/1g

1211442-87-3
5 suppliers
inquiry
Yield:1211442-87-3 54%
Reaction Conditions:
Stage #1: 5-bromo-2-chloro-N-cyclopentylaminopyrimidine-4-amine;Propiolaldehyde diethyl acetalwith triethylamine;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 in tetrahydrofuran at 20; for 0.166667 h;Inert atmosphere;
Stage #2: copper(l) iodide in tetrahydrofuran at 60; for 48 h;Product distribution / selectivity;Inert atmosphere;
Steps:
104
To a stirred solution of (5-bromo-2-chloro pyrimidin-4-yl)-cyclopentyl-amine (1.00 g, 3.62 mmol) and PdCl2(dppf)-dichloromethane (148 mg, 0.181 mmol) in THF (10 mL) is added Et3N (0.757 mL, 5.43 mmol) and 3,3-diethoxy-propyne (0.778 mL, 5.43 mmol) sequentially at room temperature. The mixture is degassed under a stream of N2 and stirred at room temperature for 10 minutes before CuI (29 mg, 0.154 mmol) is added. The reaction vessel is evacuated and back-filled with N2 (x3) and heated at 60 °C for 48 hours. The mixture is allowed to cool, diluted with EtOAc, filtered and partitioned between H2O and ethyl acetate. The phases are separated and the aqueous layer is further extracted with EtOAc (x3), combined organic extracts are dried (MgSO^, filtered and concentrated. The residue is purified by SiO2 chromatography, eluting with a gradient of 5% EtO Ac/petrol to 20% EtOAc / petrol to give [2-chloro-5-(3,3-diethoxy-prop-1-ynyl)-pyrimidin-4-yl]-cyclopentyl amine (636 mg, 54%). MS(ESI) m/z 324.2 (M+H)+
References:
WO2010/20675,2010,A1 Location in patent:Page/Page column 51; 52
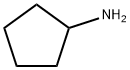
1003-03-8
235 suppliers
$10.00/1g

1211442-87-3
5 suppliers
inquiry