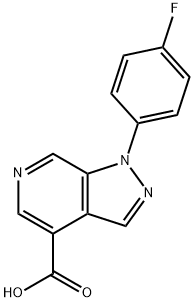
1-(4-fluorophenyl)-1H-pyrazolo[3,4-c]pyridine-4-carboxylic acid synthesis
- Product Name:1-(4-fluorophenyl)-1H-pyrazolo[3,4-c]pyridine-4-carboxylic acid
- CAS Number:1220165-92-3
- Molecular formula:C13H8FN3O2
- Molecular Weight:257.22

124-38-9
134 suppliers
$214.00/14L
![4-broMo-1-(4-fluorophenyl)-1H-pyrazolo[3,4-c]pyridine](/CAS/20130318/GIF/CB22638689.gif)
1220165-54-7
26 suppliers
$220.00/50mg
![1-(4-fluorophenyl)-1H-pyrazolo[3,4-c]pyridine-4-carboxylic acid](/CAS/20150408/GIF/CB32648230.gif)
1220165-92-3
12 suppliers
inquiry
Yield:1220165-92-3 79%
Reaction Conditions:
Stage #1: 4‐bromo‐1‐(4‐fluorophenyl)‐1H‐pyrazolo[3,4‐c]pyridinewith isopropylmagnesium chloride in tetrahydrofuran at -20 - -10;
Stage #2: carbon dioxide in tetrahydrofuran at 22;
Stage #3: with hydrogenchloride;water pH=6 - 7;
Steps:
30
To a 1 L flask was charged 4-bromo-l-(4-fluorophenyl)-lH-pyrazolo[3,4-c]pyridine (50.0 g, 171.1 mmol, 1 eq) and TΗF (300 mL). The slurry was cooled to -2O0C. i- PrMgCl solution (128.2 mL, 256.4 mmol, 2.0 M in TΗF, 1.5 eq) was charged at a rate to keep the temperature below -1O0C. The batch was held at -1O0C for 3 hours. CO2 gas was then bubbled into the reaction mixture until the temperature increase peaked, and the temperature began to drop. The temperature was adjusted to 220C, and /-PrOAc (325 mL) was added. A solution of aqueous HCl was prepared from concentrated HCl (55 rnL) and water (195 rnL). About 10 mL of this HCl solution was charged to the reaction mixture to achieve pH 6-7. The batch was then heated to 550C, and the remaining -240 mL of the HCl solution was charged. The batch was cooled to ambient temperature over 1 hour, and held at this temperature for 1 hour. The batch was then filtered, and the solid washed with water and /-PrOAc. The solid was oven dried under vacuum to afford l-(4- fluorophenyl)-lH-pyrazolo[3,4-c]pyridine-4-carboxylic acid as a yellow solid, 38.4 g, 90 wt.% purity, 79% yield.
References:
WO2010/36632,2010,A1 Location in patent:Page/Page column 155; 156
![1H-Pyrazolo[3,4-c]pyridine-4-carboxylic acid, 1-(4-fluorophenyl)-, ethyl ester](/CAS/20210305/GIF/1220165-87-6.gif)
1220165-87-6
0 suppliers
inquiry
![1-(4-fluorophenyl)-1H-pyrazolo[3,4-c]pyridine-4-carboxylic acid](/CAS/20150408/GIF/CB32648230.gif)
1220165-92-3
12 suppliers
inquiry
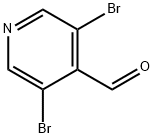
70201-42-2
210 suppliers
$5.00/1g
![1-(4-fluorophenyl)-1H-pyrazolo[3,4-c]pyridine-4-carboxylic acid](/CAS/20150408/GIF/CB32648230.gif)
1220165-92-3
12 suppliers
inquiry
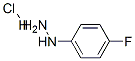
823-85-8
366 suppliers
$5.00/5g
![1-(4-fluorophenyl)-1H-pyrazolo[3,4-c]pyridine-4-carboxylic acid](/CAS/20150408/GIF/CB32648230.gif)
1220165-92-3
12 suppliers
inquiry
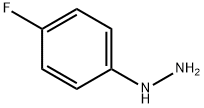
371-14-2
81 suppliers
inquiry
![1-(4-fluorophenyl)-1H-pyrazolo[3,4-c]pyridine-4-carboxylic acid](/CAS/20150408/GIF/CB32648230.gif)
1220165-92-3
12 suppliers
inquiry