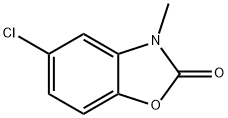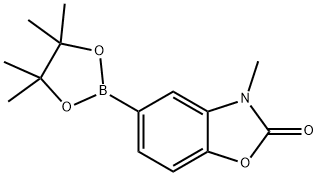
3-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2(3H)-benzoxazolone synthesis
- Product Name:3-methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2(3H)-benzoxazolone
- CAS Number:1220696-32-1
- Molecular formula:C14H18BNO4
- Molecular Weight:275.11
95-25-0
386 suppliers
$5.00/250mg

73183-34-3
587 suppliers
$6.00/5g

1220696-32-1
63 suppliers
inquiry
Yield: 97%
Reaction Conditions:
with potassium acetate;palladium diacetate;XPhos for 3 h;Reflux;Inert atmosphere;Large scale;
Steps:
2.ii
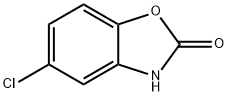
A solution of 5-chloro-3-methyl-1,3-benzoxazol-2(3H)-one (stage ii)) (350 g, 1.91 mol), B2pin2 (581.0 g, 2.29 mol) and KOAc (561.3 g, 5.72 mol) was vacuum degassed and purged with N2 (×3). Pd(OAc)2 (12.9 g, 57.2 mmol) and XPhos (54.6 g, 114 mmol) were added and the mixture was vacuum degassed and purged with N2 (×3). The mixture was heated to 75° C. A large exotherm was observed at 70° C. which warmed-up the mixture to reflux (100° C.). The reaction mixture was stirred for 1 h with no heating. HPLC analysis indicated 2.5% of the starting material remaining therefore the mixture was heated at 85° C. for 1 h. At this stage, no further change was observed. Additional portions of B2pin2 (14.6 g, 57.2 mmol), KOAc (5.7 g, 57.2 mmol), Pd(OAc)2 (12.9 g, 57.2 mmol) and XPhos (27.3 g, 57.2 mmol) were added and the mixture was stirred for 1 h at 75° C. HPLC analysis showed no starting material remaining. The mixture was cooled to rt, filtered through a pad of Celite (501 g) and the cake was washed with EtOAc (2240 ml). The filtrate was combined with two other batches prepared in the same way (2×350 g) and evaporated. This gave 1865.1 g of the product as a grey solid (97% yield, 90.0% pure by LC, 82±2% pure by 1H NMR (DMSO-d6) assay vs TCNB). 1H NMR (270 MHz, DMSO-d6): δ 7.40-7.50 (m, 2H), 7.30 (d, 1H), 3.40 (s, 3H), 1.30 (s, 12H). LCMS (5 cm_ESCI_aq. formic acid_methanol_) tR 4.91 (min) m/z 276.1 (MH+).
References:
AstraZeneca AB;Lonn, Hans Roland;Connolly, Stephen;Swallow, Steven;Karlsson, Staffan PO;Aurell, Carl-Johan;Pontén, John Fritiof;Doyle, Kevin James;Van de Poël, Amanda Jane;Jones, Graham Peter;Watson, David Wyn;MacRitchie, Jaqueline Anne;Palmer, Nicholas John US9522894, 2016, B2 Location in patent:Page/Page column 53; 54
70672-82-1
37 suppliers
$45.00/10mg

73183-34-3
587 suppliers
$6.00/5g

1220696-32-1
63 suppliers
inquiry

70672-82-1
37 suppliers
$45.00/10mg

1220696-32-1
63 suppliers
inquiry
95-85-2
401 suppliers
$13.00/25g

1220696-32-1
63 suppliers
inquiry