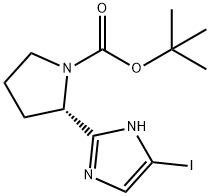
(S)-tert-butyl 2-(5-iodo-1H-imidazol-2-yl)pyrrolidine-1-carboxylate synthesis
- Product Name:(S)-tert-butyl 2-(5-iodo-1H-imidazol-2-yl)pyrrolidine-1-carboxylate
- CAS Number:1228552-62-2
- Molecular formula:C12H18IN3O2
- Molecular Weight:363.19
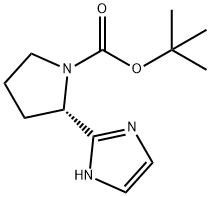
1007882-58-7
60 suppliers
$65.00/100mg

1228552-62-2
12 suppliers
inquiry
Yield:1228552-62-2 88%
Reaction Conditions:
Stage #1: (S)-tert-butyl 2-(1H-imidazol-2-yl)pyrrolidine-1-carboxylatewith N-iodo-succinimide in dichloromethane at 0; for 1.5 h;
Stage #2: with sodium sulfite in ethanol;water; for 18 h;Reflux;
Steps:
8 Synthesis of S2-4
Imidazole compound S2-3 (4.0 g, 16.8 mmol)Soluble in 100mL of dichloromethane,After cooling to 0 ° C,willN-iodosuccinimide (7.6 g, 33.6 mmol)Add to the reaction bottle in batches,Constant temperature reaction1.5 hours.After the reaction is completed, the reaction solution is washed with saturated brine.Dry over anhydrous sodium sulfate,After concentration, it was separated by column chromatography (eluent: hexane / ethyl acetate = 3/2)getWhite solid 5.3g,The yield was 64.3%.The compound (3.2 g, 6.54 mmol) was suspended in 100 mLEthanol and water (3:7)In the mixture,Sodium sulfite(7.4 g, 58 mmol) was added to the mixture and refluxed overnight (18 h).After the reaction was completed, most of the ethanol was distilled off under reduced pressure, and then 50 mL of water was added.The aqueous phase was extracted with ethyl acetate (50 mL×3).Collect organic layers,Dry over anhydrous sodium sulfate,After concentration, separation by column chromatography (eluent: hexane / ethyl acetate = 3 / l)getWhite solid (S2-4) 2.1g,The yield was 88%.
References:
CN109232612,2019,A Location in patent:Paragraph 0104; 0105; 0110; 0111

1228968-38-4
1 suppliers
inquiry

1228552-62-2
12 suppliers
inquiry
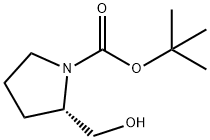
69610-40-8
398 suppliers
$6.00/5g

1228552-62-2
12 suppliers
inquiry
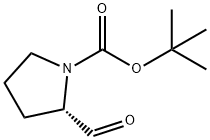
69610-41-9
262 suppliers
$9.00/1g

1228552-62-2
12 suppliers
inquiry
15761-39-4
608 suppliers
$5.00/10g

1228552-62-2
12 suppliers
inquiry