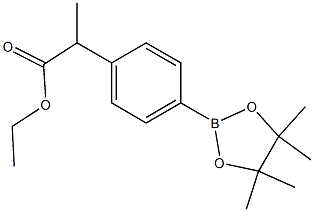
Ethyl 2-[4-(4,4,5,5-TetraMethyl-1,3,2-dioxaborolan-2-yl)phenyl]propanoate synthesis
- Product Name:Ethyl 2-[4-(4,4,5,5-TetraMethyl-1,3,2-dioxaborolan-2-yl)phenyl]propanoate
- CAS Number:1228690-28-5
- Molecular formula:C17H25BO4
- Molecular Weight:304.189
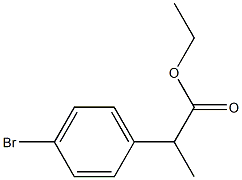
111914-79-5
22 suppliers
$258.32/0.25g

73183-34-3
587 suppliers
$6.00/5g
![Ethyl 2-[4-(4,4,5,5-TetraMethyl-1,3,2-dioxaborolan-2-yl)phenyl]propanoate](/CAS/20150408/GIF/1228690-28-5.gif)
1228690-28-5
12 suppliers
inquiry
Yield:1228690-28-5 85%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in 1,4-dioxane at 110; for 16 h;Inert atmosphere;Sealed tube;
Steps:
11.B Intermediate 11B ethyl 2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)propanoate
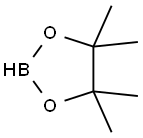
Intermediate 11B ethyl 2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)propanoate [0520] [0521] To a mixture of Intermediate 11A (120 mg, 0.467 mmol), bis(pinacolato)diboron (142 mg, 0.56 mmol), and potassium acetate (137 mg, 1.40 mmol) in dioxane (4 mL), was added PdCl2(dppf) CH2Cl2 adduct (10 mg, 0.014 mmol). The reaction mixture was degassed (3× vacuum/Ar), sealed and heated at 110° C. for 16 h. The reaction mixture was concentrated and the residue was purified by flash chromatography (0-30% EtOAc/hexane gradient) to afford 120 mg (85%) of Intermediate 11B as a yellow oil. [0522] MS (ESI) m/z: 327.2 (M+H)+; 1H NMR (500 MHz, CDCl3) δ 7.81-7.75 (m, J=8.3 Hz, 2H), 7.35-7.29 (m, J=8.0 Hz, 2H), 4.11 (dddd, J=17.8, 10.6, 7.1, 3.6 Hz, 2H), 3.77-3.66 (m, 1H), 1.49 (d, J=7.2 Hz, 3H), 1.37-1.30 (m, 12H), 1.19 (t, J=7.2 Hz, 3H)
References:
US2014/206686,2014,A1 Location in patent:Paragraph 0520-0522

111914-79-5
22 suppliers
$258.32/0.25g
25015-63-8
216 suppliers
$22.39/5G
![Ethyl 2-[4-(4,4,5,5-TetraMethyl-1,3,2-dioxaborolan-2-yl)phenyl]propanoate](/CAS/20150408/GIF/1228690-28-5.gif)
1228690-28-5
12 suppliers
inquiry
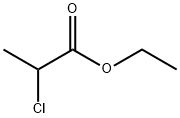
535-13-7
223 suppliers
$10.00/5g
![2-(4-BROMO-PHENYL)-4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLANE](/CAS/GIF/68716-49-4.gif)
68716-49-4
141 suppliers
$5.00/250mg
![Ethyl 2-[4-(4,4,5,5-TetraMethyl-1,3,2-dioxaborolan-2-yl)phenyl]propanoate](/CAS/20150408/GIF/1228690-28-5.gif)
1228690-28-5
12 suppliers
inquiry
14062-25-0
254 suppliers
$6.00/5g
![Ethyl 2-[4-(4,4,5,5-TetraMethyl-1,3,2-dioxaborolan-2-yl)phenyl]propanoate](/CAS/20150408/GIF/1228690-28-5.gif)
1228690-28-5
12 suppliers
inquiry