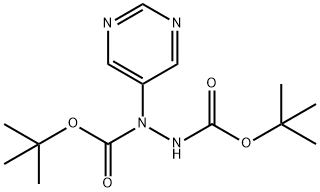
di-tert-butyl 1-(5-pyrimidinyl)-1,2-hydrazinedicarboxylate synthesis
- Product Name:di-tert-butyl 1-(5-pyrimidinyl)-1,2-hydrazinedicarboxylate
- CAS Number:1234615-84-9
- Molecular formula:C14H22N4O4
- Molecular Weight:310.35
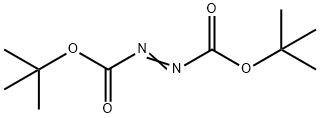
870-50-8
336 suppliers
$5.00/1g
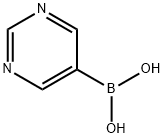
109299-78-7
303 suppliers
$10.00/1g

1234615-84-9
13 suppliers
inquiry
Yield:-
Reaction Conditions:
with copper diacetate in methanol at 60; for 1 h;
Steps:
2 Step 2: 5-Hydrazinylpyrimidine hydrochloride (A2)
In a pressure vessel pyrimidine-5-boronic acid (10 g, 80.7 mmol, 1eq.), di-tert-butyl- azodicarboxylate (18.6 g, 80.7 mmol, 1 eq.), anhydrous copper(ll)acetate (0.5 g, 2.7 mmol, 0.033 eq) in dry methanol (320ml_) were heated at 60° C for 1h. The mixture was concentrated in vacuum. The residue was solved in diethyl ether and washed with sat. aq. NaHCO3-so|. and brine. The organic layer was dried over Na2SO4 and concentrated under reduced pressure to yield the intermediate di-tert-butyl 1- (pyrimidin-5-yl)hydrazine-1 ,2-dicarboxylate. The intermediate was solved in dioxane (130 ml_) and 4M HCI in dioxane (150 mL, 592 mmol, 10.5 eq.) was slowly added. After 30 h at RT, the solid was filtered off and washed twice with diethyl ether. The solid was dried in vacuum yielding A2 (9.3 g, 79%). 1H NMR (300 MHz, DMSO, 300 K) δ [ppm] 10.43 (br.s, 1 H), 9.05 (br.s, 3H), 8.78 (s, 1 H), 8.58 (s, 2H). MS (ES) C4H6N4 requires: 110, found: 111 (M+H)+.
References:
WO2014/194975,2014,A1 Location in patent:Page/Page column 31