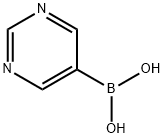
5-Pyrimidinylboronic acid synthesis
- Product Name:5-Pyrimidinylboronic acid
- CAS Number:109299-78-7
- Molecular formula:C4H5BN2O2
- Molecular Weight:123.91
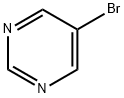
4595-59-9

109299-78-7
General procedure for the synthesis of 5-pyrimidinylboronic acid from 5-bromopyrimidine: BuLi (3.02 mL, 2M hexane solution, 7.55 mmol) was slowly added dropwise to a stirring anhydrous toluene (16 mL) and anhydrous THF (4 mL) in a mixed solution of 5-bromopyrimidine (1 g, 6.29 mmol) and triisopropyl borate (1.46 mL, 7.55 mmol), under nitrogen protection. mL) in a mixed solution at a reaction temperature of -70 °C. The reaction mixture was continuously stirred at -70 °C for 30 min and then removed from the cold bath. When the internal temperature rose to -20 °C, the reaction was quenched by dropwise addition of 2M HCl (10 mL). Phase separation was carried out after gradually warming the mixture to room temperature. The aqueous phase was adjusted to pH 5.5 with 2M KOH and extracted with THF (3 x 25 mL). The organic phases were combined, dried with MgSO4, filtered and concentrated under reduced pressure to give a colorless solid. The solid was slurried in acetonitrile (2 mL), collected by filtration and dried over a sintered mass to give the final target product 5-pyrimidinylboronic acid as a bright white solid (340 mg, 44% yield).8H (MeOD; 250 MHz) 8.98 (2H, s), 9.14 (1H, s).

4595-59-9
467 suppliers
$6.00/1g

109299-78-7
301 suppliers
$10.00/1g
Yield:109299-78-7 44%
Reaction Conditions:
Stage #1:5-bromopyrimidine with n-butyllithium;Triisopropyl borate in tetrahydrofuran;hexane;toluene at -70 - -20; for 0.5 h;
Stage #2: with hydrogenchloride;water in tetrahydrofuran;hexane;toluene at -20 - 20;
Stage #3: with potassium hydroxide in water; pH=5.5
Steps:
5-Pyrimidineboronic acid; "BuLi (3.02 ml, 2M solution in hexane, 7.55 mmol) was added dropwise to a stirring solution of 5-bromopyrimidine (1 g, 6.29 mmol) and triisopropylborate (1.46 ml, 7.55 mmol) in anhydrous toluene (16 ml) and anhydrous THF (4 ml) at-70°C under a nitrogen atmosphere. The reaction mixture was stirred at-70°C for 30 mins and then removed from the cold bath. When the internal temp. reached-20°C, the reaction was quenched by the dropwise addition of 2M HCI (10 ml). The mixture was allowed to warm to RT and then separated. The aqueous phase was taken to pH 5.5 with 2M KOH and extracted into THF (3 x 25 ml). The combined organic extracts were dried (MgS04), filtered and concentrated in vacuo to provide a colourless solid. The solid was slurried in acetonitrile (2 ml), collected by filtration and dried on the sinter to give the target boronic acid as a brilliant white solid (340 mg, 44%). 8H(MeOD; 250MHz) 8.98 (2H, s), 9.14 (1H, s).
References:
ARAKIS LTD. WO2005/103019, 2005, A1 Location in patent:Page/Page column 16

4595-59-9
467 suppliers
$6.00/1g

121-43-7
382 suppliers
$13.00/25mL

109299-78-7
301 suppliers
$10.00/1g