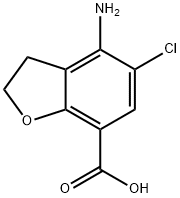
4-amino-5-chloro-2,3-dihydrobenzofuran-7-carboxylic acid synthesis
- Product Name:4-amino-5-chloro-2,3-dihydrobenzofuran-7-carboxylic acid
- CAS Number:123654-26-2
- Molecular formula:C9H8ClNO3
- Molecular Weight:213.62
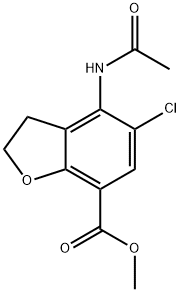
143878-29-9
110 suppliers
$15.00/100mg

123654-26-2
190 suppliers
$12.00/100mg
Yield:123654-26-2 89.8%
Reaction Conditions:
with 1-methoxy-2-propanol;water;sodium hydroxide at 90; for 8 h;
Steps:
7 Example 7 Synthesis of 4-amino-5-chloro-2,3-dihydrobenzofuran-7-carboxylic acid
The resulting intermediate 7 (30 g, 0.11 mol), water (150 mL),Propylene glycol monomethyl ether (36mL) and 50% sodium hydroxide solution (90mL), slowly heated to 90 ° C with stirring, the same temperature for 8h, the reaction was monitored by TLC. After the reaction is completed,Cooled to 5 ° C with ice bath and stirred for 2h, filtered with suction,The filter cake was washed with an appropriate amount of water added to 500mL single-necked flask,Water (210 mL) and propylene glycol monomethyl ether (35 mL) were added,The temperature of the reaction solution was raised to 80 ° C and the reaction solution became homogeneous.Dilute dilute hydrochloric acid, adjust pH to about 3, precipitated solid.After cooling to 10 ° C, stirring was continued for 2h. Suction filtration,The filter cake was washed with an appropriate amount of water and dried to give an off-white solid,Yield 20.8g, yield 89.8%, purity 99%
References:
CN107337658,2017,A Location in patent:Paragraph 0051; 0052
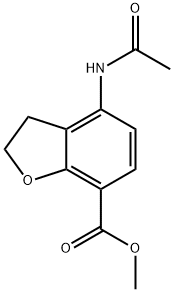
149466-67-1
29 suppliers
inquiry

123654-26-2
190 suppliers
$12.00/100mg
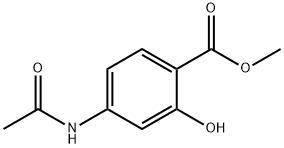
4093-28-1
149 suppliers
$6.00/5g

123654-26-2
190 suppliers
$12.00/100mg

202664-85-5
15 suppliers
inquiry

123654-26-2
190 suppliers
$12.00/100mg
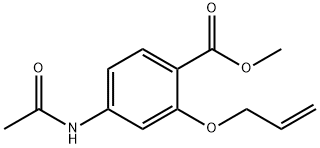
91958-13-3
1 suppliers
inquiry

123654-26-2
190 suppliers
$12.00/100mg