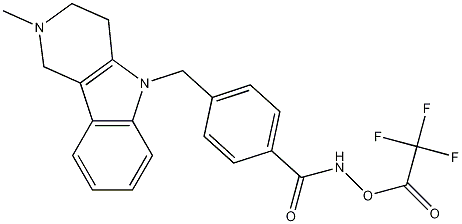
N-Hydroxy-4-[(1,2,3,4-tetrahydro-2-methyl-5H-pyrido[4,3-b]indol-5-yl)methyl]benzamide 2,2,2-Trifluoroacetate synthesis
- Product Name:N-Hydroxy-4-[(1,2,3,4-tetrahydro-2-methyl-5H-pyrido[4,3-b]indol-5-yl)methyl]benzamide 2,2,2-Trifluoroacetate
- CAS Number:1239262-52-2
- Molecular formula:C22H22F3N3O4
- Molecular Weight:431.4077
![4-(2-Methyl-l,2^,4-tetrahydro-pyrido[4,3-6]indol-5-ylmethyl)beiizoic acid methyl ester](/CAS/20180713/GIF/1239034-70-8.gif)
1239034-70-8
28 suppliers
$22.00/100mg
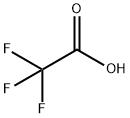
76-05-1
673 suppliers
$9.00/1g
![N-Hydroxy-4-[(1,2,3,4-tetrahydro-2-methyl-5H-pyrido[4,3-b]indol-5-yl)methyl]benzamide 2,2,2-Trifluoroacetate](/CAS2/GIF/1239262-52-2.gif)
1239262-52-2
86 suppliers
$32.00/1mg
Yield: 31%
Reaction Conditions:
Stage #1:4-(2-methyl-1,2,3,4-tetrahydro-pyrido[4,3-b]indol-5-ylmethyl)benzoic acid methyl ester with hydroxylamine hydrochloride;sodium methylate in methanol at 20; for 24 h;Inert atmosphere;
Stage #2:trifluoroacetic acid in waterHPLC column;
Steps:
[0222] N-Hydroxy-4-(2-methyl-l,2,3,4-tetrahydro-pyrido[4,3-b]indol-5- ylmethyl)benzamide, trifluoroacetic acid salt (6): 4-(2 -Methyl- 1,2,3,4-tetrahydro- pyrido[4,3-]indol-5-ylmethyl)benzoie acid methyl ester (20) (0.50 g, 1.5 mmol) and hydroxylamine hydrochloride (0.62 g, 9.0 mmol) were placed under argon and dissolved in 5 mL of methanol. To it was added a 25% sodium methoxide solution in methanol (2.6 g, 12 mmol) which resulted in the formation of a white precipitate. The reaction was stirred for 24 h after which the reaction was diluted with ethyl acetate (20 mL) and saturated sodium bicarbonate (20 mL). The organic layer was isolated and the aqueous layer was further extracted with ethyl acetate (2 x 10 mL). The combined organic layers were washed with brine (10 mL), dried (Na2SO4) and concentrated in vacuo. The crude extract was purified by HPLC to yield the title compound (TFA salt, 0.21 g, 31%) as a white solid. 1H NMR (400 MHz, DMSO): S 11.17 (br, IH), 10.17 (br, IH), 9.00 (br, IH), 7.67 (d, 2H, J= 7.9 Hz), 7.48 (t, 2H, J= 7.9 Hz), 7.17-7.06 (m, 4H), 5.44 (br, 2H), 4.50 (br, 2H), 3.60 (br, 2H), 3.10 (m, 2H), 3.00 (s, 3H). 13C NMR APT (100 MHz, MeOD): δ 141.5 (up), 137.0 (up), 132.3 (up), 131.7 (up), 127.7 (down), 126.9 (down), 124.8 (up), 122.3 (down), 120.2 (down), 118.2 (down), 110.6 (down), 102.7 (up), 51.0 (up), 50.6 (up), 46.0 (up), 42.3 (down), 20.1 (up). ESI-HRMS (m/z): [M+H]+ calcd. for C20H21N3O2, 336.1707; found, 336.1708. Analytical HPLC: Purity = 100%, tR= 5.71 min, Method A.
References:
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS;KOZIKOWSKI, Alan;BUTLER, Kyle, B.;KALIN, Jay, Hans WO2011/11186, 2011, A2 Location in patent:Page/Page column 54-55
1445-73-4
467 suppliers
$15.00/25g
![N-Hydroxy-4-[(1,2,3,4-tetrahydro-2-methyl-5H-pyrido[4,3-b]indol-5-yl)methyl]benzamide 2,2,2-Trifluoroacetate](/CAS2/GIF/1239262-52-2.gif)
1239262-52-2
86 suppliers
$32.00/1mg
100-63-0
356 suppliers
$10.00/1g
![N-Hydroxy-4-[(1,2,3,4-tetrahydro-2-methyl-5H-pyrido[4,3-b]indol-5-yl)methyl]benzamide 2,2,2-Trifluoroacetate](/CAS2/GIF/1239262-52-2.gif)
1239262-52-2
86 suppliers
$32.00/1mg
![2-methyl-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indole](/CAS/GIF/5094-12-2.gif)
5094-12-2
60 suppliers
$30.00/100mg
![N-Hydroxy-4-[(1,2,3,4-tetrahydro-2-methyl-5H-pyrido[4,3-b]indol-5-yl)methyl]benzamide 2,2,2-Trifluoroacetate](/CAS2/GIF/1239262-52-2.gif)
1239262-52-2
86 suppliers
$32.00/1mg
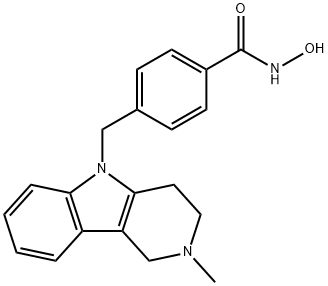
1252003-15-8
92 suppliers
$35.00/5mg

76-05-1
673 suppliers
$9.00/1g
![N-Hydroxy-4-[(1,2,3,4-tetrahydro-2-methyl-5H-pyrido[4,3-b]indol-5-yl)methyl]benzamide 2,2,2-Trifluoroacetate](/CAS2/GIF/1239262-52-2.gif)
1239262-52-2
86 suppliers
$32.00/1mg