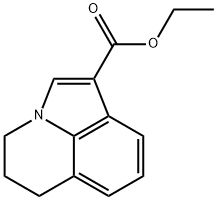
ETHYL 2,3-DIHYDRO-1H-PYRROLO[3,2,1-IJ]QUINOLINE-6-CARBOXYLATE synthesis
- Product Name:ETHYL 2,3-DIHYDRO-1H-PYRROLO[3,2,1-IJ]QUINOLINE-6-CARBOXYLATE
- CAS Number:124730-53-6
- Molecular formula:C14H15NO2
- Molecular Weight:229.27

14164-86-4
0 suppliers
inquiry
![ETHYL 2,3-DIHYDRO-1H-PYRROLO[3,2,1-IJ]QUINOLINE-6-CARBOXYLATE](/CAS/GIF/124730-53-6.gif)
124730-53-6
19 suppliers
inquiry
Yield:124730-53-6 48%
Reaction Conditions:
with magnesium chloride in tetrahydrofuran;2-methoxy-ethanol; for 6 h;Heating / reflux;
Steps:
I Preparation I 5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinoline; 5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinoline-1-carboxylic acid ethyl ester
Preparation I 5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinoline 3-(3,4-Dihydro-2H-quinolin-1-yl)-2-oxopropionic acid ethyl ester [0144] To a solution of 1,2,3,4-tetrahydroquinoline (75.5 mL, 0.59 mol) in tetrahydrofuran (300 mL) was added bromoethyl pyruvate (40 mL, 0.29 mol) dropwise over 30 minutes. Following 24 hours of stirring, the reaction mixture was filtered, the filter cake rinsed well with tetrahydrofuran (100 mL) and the filtrate concentrated under reduced pressure to dryness to give 79.7 g of the desired compound as a red oil. [0145] 5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinoline-1-carboxylic acid ethyl ester [0146] Magnesium chloride (27.7 g, 0.29 mol) was added to 2-methoxyethanol (400 mL) and the mixture heated to reflux. A solution of 3-(3,4-Dihydro-2H-quinolin-1-yl)-2-oxopropionic acid ethyl ester (0.29 mol) in 2-methoxyethanol (100 mL) and tetrahydrofuran (40 mL) was slowly added to the MgCl2 mixture over 1 hour. Upon completion of addition, the mixture was stirred for 5 hours at reflux, and then concentrated in vacuo. The concentrated crude mixture was treated with 2N hydrochloric acid (500 mL) and extracted with dichloromethane (3×400 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated in vacuo. The residue was subjected to silica gel chromatography, eluting with 20% ethyl acetate/hexanes. Fractions containing product were combined and concentrated under reduced pressure to provide 31.6 g (48%) of the desired compound as an orange solid. [0147] MS (IS, m/z) C14H15NO2 (M++1)=230. [0148] 5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinoline-1-carboxylic acid [0149] To a solution of 5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinoline-1-carboxylic acid ethyl ester (31 g, 0.14 mol) in ethanol (200 mL) and water (70 mL) was added 5 N aqueous sodium hydroxide (60 mL, 0.3 mol) and the resulting mixture stirred at reflux for 3 hours. The reaction mixture was cooled to 20-24° C., diluted with water (2 L) and washed with dichloromethane (2×200 mL) and diethyl ether (1×200 mL). The aqueous layer was filtered through Celite and the filtrate was acidified with conc. HCl (25 mL) to precipitate the product. The solid was filtered, washed with water (200 mL), and dried in vacuo to give 23.2 g (85%) of the desired compound as a light yellow solid. [0150] MS (IS, m/z) C12H11NO2 (M++1)=202. [0151] Decarboxylation [0152] To a solution of 5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinoline-1-carboxylic acid (3.7 g, 18.4 mmol) in 20 mL of quinoline was added copper chromite (1.5 g, 4.8 mmol). The resulting mixture was stirred at 185° C. for 4 hours and then cooled to 20-24° C., diluted with dichloromethane (100 mL) and filtered through Celite. The filtrate was then washed sequentially with 2 N hydrochloric acid (2×50 mL) and 2 N aqueous sodium hydroxide (25 mL). The remaining organic phase was concentrated in vacuo. The residue was subjected to silica gel chromatography, eluting with 5% EtOAc/Hexanes. Fractions containing product were combined and concentrated under reduced pressure to provide 1.67 g (58%) of the desired compound as a light tan solid. [0153] 1H NMR (400 MHz, DMSO-d6) δ 7.31-7.29 (d, 1H, J=7.8 Hz), 7.28-7.27 (d, 1H, J=2.93 Hz), 6.9-6.86 (t, 1H, J=7.6 Hz), 6.82-6.8 (dd, 1H, J=6.8, 1.0 Hz), 6.33-6.32 (d, 1H, J=2.93 Hz), 4.15-4.12 (t, 2H, J=5.6 Hz), 2.92-2.89 (t, 2H, J=6.1 Hz), 2.15-2.08 (m, 2H).
References:
US2003/229026,2003,A1 Location in patent:Page 15
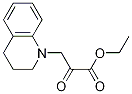
152712-44-2
6 suppliers
inquiry
![ETHYL 2,3-DIHYDRO-1H-PYRROLO[3,2,1-IJ]QUINOLINE-6-CARBOXYLATE](/CAS/GIF/124730-53-6.gif)
124730-53-6
19 suppliers
inquiry
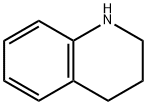
635-46-1
367 suppliers
$5.00/10g
![ETHYL 2,3-DIHYDRO-1H-PYRROLO[3,2,1-IJ]QUINOLINE-6-CARBOXYLATE](/CAS/GIF/124730-53-6.gif)
124730-53-6
19 suppliers
inquiry
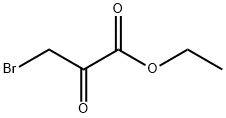
70-23-5
317 suppliers
$6.00/5g
![ETHYL 2,3-DIHYDRO-1H-PYRROLO[3,2,1-IJ]QUINOLINE-6-CARBOXYLATE](/CAS/GIF/124730-53-6.gif)
124730-53-6
19 suppliers
inquiry