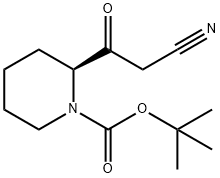
(S)-tert-butyl 2-(2-cyanoacetyl)piperidine-1-carboxylate synthesis
- Product Name:(S)-tert-butyl 2-(2-cyanoacetyl)piperidine-1-carboxylate
- CAS Number:1260587-50-5
- Molecular formula:C13H20N2O3
- Molecular Weight:252.31

200184-53-8
25 suppliers
$152.00/250mg

75-05-8
1049 suppliers
$10.00/10g

1260587-50-5
7 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: acetonitrilewith sodium bis(trimethylsilyl)amide in tetrahydrofuran;hexanes at -78 - -40;
Stage #2: (S)-1-tert-butyl 2-methyl piperidine-1,2-dicarboxylate in tetrahydrofuran;hexanes at -78 - -40;
Stage #3: with acetic acid in tetrahydrofuran;hexanes at -78 - 20;
Steps:
Intermediate 4 :To a solution of acetonitrile (5 ml, 93.8 mmol) in dry THF (50 ml) at -78 °C was added dropwise NaN(TMS)2 (34 ml, 68 mmol, 2M in hexanes). The solution was warmed up to -40 °C and stirred for 20 min. The solution was then cooled down to -78 °C and a solution of the ester (Intermediate 2) (7.6 g, 31.1 mmol) in THF (20 ml) was added dropwise. The solution was warmed up to -40 °C and stirred for 2 h. The solution was then cooled down to -78 °C and a solution of acetic acid (4.8 ml, 80 mmol) in THF (20 ml) added dropwise. The solution was then warmed to RT and volatiles were removed under reduced pressure at 40 °C. The resulting residue was dissolved in EtOAc (300 mL) and the organic phase was washed 2x each with brine. Volatiles were removed under reduced pressure at 40 °C.1H NMR (DMSO, 300 MHz) δ 4.63 (br s, 1H), 4.18-4.13 (m, 1H), 3.82-3.78 (m, 1H), 3.65 (s, 2H), 2.85-2.63 (m, 1H), 1.65-1.52 (m, 9H), 1.38 (s, 9H).LCMS m/z : 153 [M-Boc group+H], tR = 2.50 min.
References:
WO2011/163518,2011,A1 Location in patent:Page/Page column 153

75-05-8
1049 suppliers
$10.00/10g
![1-BOC-(2S)-[N-METHOXY-N-METHYLCARBAMOYL]PIPERIDINE](/CAS/GIF/203056-27-3.gif)
203056-27-3
17 suppliers
$110.00/1g

1260587-50-5
7 suppliers
inquiry
26250-84-0
283 suppliers
$9.00/5g

1260587-50-5
7 suppliers
inquiry