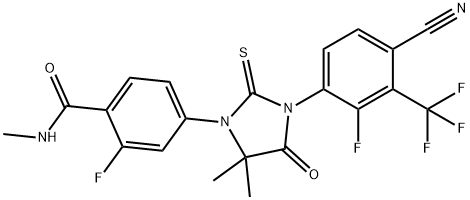
Benzamide, 4-[3-[4-cyano-2-fluoro-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-thioxo-1-imidazolidinyl]-2-fluoro-N-methyl- synthesis
- Product Name:Benzamide, 4-[3-[4-cyano-2-fluoro-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-thioxo-1-imidazolidinyl]-2-fluoro-N-methyl-
- CAS Number:1272719-00-2
- Molecular formula:C21H15F5N4O2S
- Molecular Weight:482.43
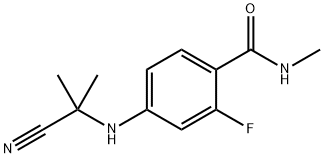
915087-32-0
102 suppliers
inquiry
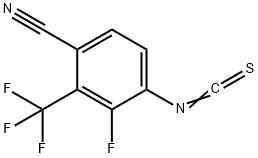
1311158-94-7
13 suppliers
inquiry
![Benzamide, 4-[3-[4-cyano-2-fluoro-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-thioxo-1-imidazolidinyl]-2-fluoro-N-methyl-](/CAS/20210111/GIF/1272719-00-2.gif)
1272719-00-2
0 suppliers
inquiry
Yield:1272719-00-2 33%
Reaction Conditions:
Stage #1: N-methyl-2-fluoro-4-(1,1-dimethyl-cyanomethyl)-aminobenzamide;C9H2F4N2S in N,N-dimethyl-formamide at 110; for 12 h;Microwave irradiation;
Stage #2: hydrogenchloride in ethanol;N,N-dimethyl-formamide; for 1 h;Reflux;
Steps:
12
Synthesis of 4-[3-(4-Cyano-2-fluoro-3-trifluoromethyl-phenyl)-5,5-dimethyl-4-oxo-2-thioxo- imidazolidin-l-yl]-2-fluoro-N-methyl-benzamide (Example 12)3b 6b Example 12[00208] A mixture of 3b (492mg, 2mmol) and 6b (470 mg, 2mmol) in DMF (lOmL) was heated under microwave irradiation at 110°C for 12 h. To this mixture was added ethanol (lOmL) and aqueous HCl solution (2N, 5mL). The resulting mixture was heated at reflux for lh. The solution was poured into ice-cold water and extracted with ethyl acetate (3x3 OmL). The organic layers were combined, dried over MgS04, and concentrated in vacuo. The residue was purified with silica gel column chromatography using petroleum ether : ethyl acetate (2:1), affording the title compound (Example 12, 320 mg, 33% yield). 1H NMR (CDC13, 500MHz): δ 8.28 (1H, dd, J=8.6, 3.5), 7.80 (2H, dd, J=15.6, 8.0), 7.26 (1H, m), 7.18 (1H, dd, J=12.0, 2.0Hz), 6.7 (1H, br s), 3.00 (3H, d, J=5 Hz), 1.53 (6H,s). MS (ES-API positive): 483 (M+H)+.
References:
WO2011/29392,2011,A1 Location in patent:Page/Page column 70
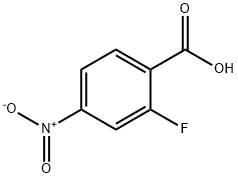
403-24-7
369 suppliers
$5.00/250mg
![Benzamide, 4-[3-[4-cyano-2-fluoro-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-thioxo-1-imidazolidinyl]-2-fluoro-N-methyl-](/CAS/20210111/GIF/1272719-00-2.gif)
1272719-00-2
0 suppliers
inquiry
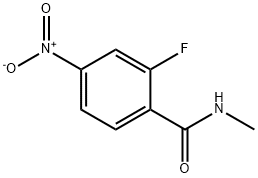
915087-24-0
130 suppliers
$220.00/1g
![Benzamide, 4-[3-[4-cyano-2-fluoro-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-thioxo-1-imidazolidinyl]-2-fluoro-N-methyl-](/CAS/20210111/GIF/1272719-00-2.gif)
1272719-00-2
0 suppliers
inquiry
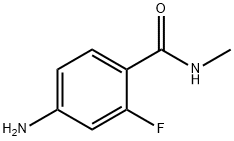
915087-25-1
236 suppliers
$8.00/250mg
![Benzamide, 4-[3-[4-cyano-2-fluoro-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-thioxo-1-imidazolidinyl]-2-fluoro-N-methyl-](/CAS/20210111/GIF/1272719-00-2.gif)
1272719-00-2
0 suppliers
inquiry