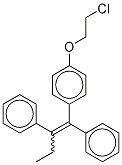
(E/Z)-1-[4-(2-Chloroethoxy)phenyl]-2-(4-hydroxyphenyl)-1-phenyl-1-butene synthesis
- Product Name:(E/Z)-1-[4-(2-Chloroethoxy)phenyl]-2-(4-hydroxyphenyl)-1-phenyl-1-butene
- CAS Number:1276031-01-6
- Molecular formula:C24H23ClO2
- Molecular Weight:378.89
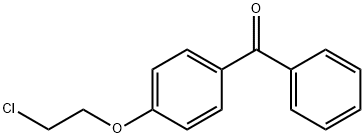
3439-73-4
14 suppliers
$495.22/5MG
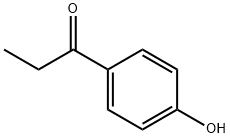
70-70-2
476 suppliers
$13.00/25g
![(E/Z)-1-[4-(2-Chloroethoxy)phenyl]-2-(4-hydroxyphenyl)-1-phenyl-1-butene](/CAS/GIF/1276031-01-6.gif)
1276031-01-6
5 suppliers
$495.00/50mg
Yield:1276031-01-6 44%
Reaction Conditions:
with titanium tetrachloride;zinc in tetrahydrofuran at 0;Inert atmosphere;Reflux;McMurry reaction;
Steps:
(Z)-2-(4-Hydroxylphenyl)-1-[4-[2-(4-methylpiperazin-1-yl)ethoxy]phenyl]-1-phenylbut-1-ene [(Z)-11a]:
Titanium tetrachloride (1.97 mL, 18 mmol) was added drop wise to a stirred suspension of Zn powder (2.35 g, 36 mmol) in dry THF (30 mL) under an argon atmosphere at -10 °C, and this mixture was heated at reflux for 1.5 h to produce the titanium reagent. A cooled suspension of this titanium reagent was added to a solution of 4-(2-chloroethoxy)benzophenone (5a, 1.56 g, 6.0 mmol) and 4-hydroxypropiophenone (9, 0.9 g, 6.0 mmol) in THF (40 mL) at 0 °C, and the reaction was allowed to proceed at reflux for 2 h. After cooling to 25 °C, the reaction mixture was poured into a 10% aqueous K2CO3 solution (90 mL), this mixture was stirred vigorously for 5 min, and the dispersed insoluble material was removed by vacuum filtration. The organic fraction was separated, the aqueous layer was extracted with EtOAc (3 x 50 mL), and the combined organic fractions were dried (Na2SO4). Removal of the solvent in vacuo gave a residue which was purified by silica gel column chromatography using EtOAc-hexane (1:4, v/v) as eluent to furnish a mixture of the (Z)-10a and (E)-10b stereoisomers in a ratio of 1:1 (1H NMR integrals) in 44% yield (1.0 g); mp 136-138 °C. All attempts to separate these (Z)-10a and (E)-10b stereoisomers by fractional crystallization from solvents of different polarity (diethyl ether, ethyl acetate, isopropanol and ethanol) were unsuccessful. Therefore, this mixture of (Z)-10a and (E)-10b, without separation, was added to a solution of N-methylpiperazine (26.4 g, 264 mmol) in ethanol (50 mL). The mixture was heated under reflux for 24 h, the solvent was evaporated in vacuo, and the residue obtained was purified by silica gel column chromatography using methanol-chloroform (1:9, v/v) as eluent to give a 1:1 (1H NMR integrals) of the (Z)-11a and (E)-11b stereoisomers in 90% yield (1.05 g). Repeated fractional crystallization of this mixture from methanol furnished 170 mg of (Z)-11a as a white solid;
References:
Abdellatif, Khaled R.A.;Velázquez, Carlos A.;Huang, Zhangjian;Chowdhury, Morshed A.;Knaus, Edward E. [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 4,p. 1195 - 1198] Location in patent:experimental part