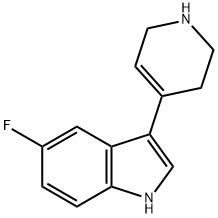
5-FLUORO-3-(1,2,3,6-TETRAHYDRO-PYRIDIN-4-YL)-1H-INDOLE synthesis
- Product Name:5-FLUORO-3-(1,2,3,6-TETRAHYDRO-PYRIDIN-4-YL)-1H-INDOLE
- CAS Number:127626-06-6
- Molecular formula:C13H13FN2
- Molecular Weight:216.25
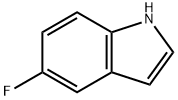
399-52-0
381 suppliers
$10.00/1g
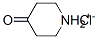
41979-39-9
0 suppliers
$15.00/10mg

127626-06-6
18 suppliers
$90.00/100mg
Yield:127626-06-6 95%
Reaction Conditions:
with natrium in methanol; for 18 h;Heating / reflux;
Steps:
2.a
To a solution of sodium (60 g, 2.6 mol) in 1000 ml of methanol was added 5-fluoroindole (49 g, 0.36 mol) and 4-piperidone. H2O.HCl (170 g, 1.11 mol). The mixture was heated under reflux for 18 h, then concentrated, water was added and extracted with ethyl acetate. The combined organic layer was dried over sodium sulfate and then concentrated. The resulting solid was dissolved in methanol (about 200 ml) and then diluted with water (about 1000-1500 ml). The precipitate was collected, washed with water and petroleum ether and then dried in a vacuum oven at 60° C. Yield 74 g (95%) of a yellow solid.
References:
US2006/122206,2006,A1 Location in patent:Page/Page column 3

399-52-0
381 suppliers
$10.00/1g

127626-06-6
18 suppliers
$90.00/100mg

399-52-0
381 suppliers
$10.00/1g
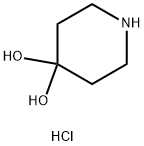
40064-34-4
0 suppliers
$10.00/5g

127626-06-6
18 suppliers
$90.00/100mg
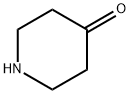
41661-47-6
0 suppliers
$45.00/250mg

399-52-0
381 suppliers
$10.00/1g

127626-06-6
18 suppliers
$90.00/100mg