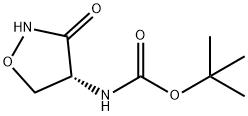
Carbamic acid, [(4R)-3-oxo-4-isoxazolidinyl]-, 1,1-dimethylethyl ester (9CI) synthesis
- Product Name:Carbamic acid, [(4R)-3-oxo-4-isoxazolidinyl]-, 1,1-dimethylethyl ester (9CI)
- CAS Number:128346-20-3
- Molecular formula:C8H14N2O4
- Molecular Weight:202.21
Yield:128346-20-3 157 g
Reaction Conditions:
with triethylamine in tetrahydrofuran;water at 10;
Steps:
1 Example 1: tert-butyl N- [(4R)-3-oxoisoxazolidin-4-yll carbamate
30 D-cycloserine (100 g) was dissolved in tetrahydrofuran (1000 ml) and water (1000 ml) then triethylamine (144 ml) was added. The stuffed solution was cooled to 10°C then a solution of ditert-butylcarbonate (224 g) in tetrahydrofuran (1000 ml) was added dropwise over a period of 1hour and the resulting reaction mixture was stirred overnight at room temperature.The solventwas removed in vacuo then the residue was acidified to pH 2-3 with a 2N hydrochloric acid solution. A precipitate was obtained, which was filtered to obtain a white solid. The filtrate was extracted with dichloromethane (6*200 ml). The combined organic layers were dried oversodium sulphate and the solvent evaporated in vacuo.The combined solids were triturated with diethyl ether then filtered and rinsed with cold diethyl ether and dried to afford the title product as a white solid (157 g). ‘H-NMR (CDC13, 400 MHz): 5.20 (m, 1H), 4.80 (m, 1H), 4.60 (m, 1H), 4.10 (m, 1H), 1.50 (s, 9H).
References:
WO2013/50302,2013,A1 Location in patent:Page/Page column 119; 220

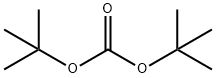
24424-99-5
814 suppliers
$13.50/25G
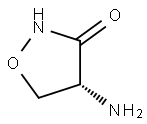
68-41-7
531 suppliers
$5.00/100mg
![Carbamic acid, [(4R)-3-oxo-4-isoxazolidinyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/128346-20-3.gif)
128346-20-3
3 suppliers
inquiry