
(1R,4R)-[4-(3-Nitro-pyridin-2-ylaMino)-cyclohexyl]-carbaMic acid tert-butyl ester synthesis
- Product Name:(1R,4R)-[4-(3-Nitro-pyridin-2-ylaMino)-cyclohexyl]-carbaMic acid tert-butyl ester
- CAS Number:1289386-01-1
- Molecular formula:C16H24N4O4
- Molecular Weight:336.39
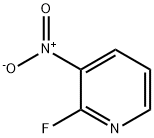
1480-87-1
208 suppliers
$6.00/1g
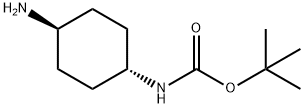
177906-48-8
215 suppliers
$6.00/250mg
![(1R,4R)-[4-(3-Nitro-pyridin-2-ylaMino)-cyclohexyl]-carbaMic acid tert-butyl ester](/CAS/20120318/GIF/CB62574899.gif)
1289386-01-1
7 suppliers
inquiry
Yield:1289386-01-1 65%
Reaction Conditions:
with triethylamine in acetonitrile at 85; for 12 h;
Steps:
10.1 (1) Preparation of intermediate XI
1.5 g of 2-fluoro-3-nitropyridine,1.5 g of tert-butyl trans-(4-aminocyclohexyl)carbamate was dissolved in acetonitrile.4 mL of triethylamine was added and reacted at 85 ° C for 12 hours.The reaction solution was cooled to room temperature and concentrated under reduced pressure.Adding an aqueous solution of citric acid, heating and stirring at 30 ° C, and vacuum filtration.The filter cake was washed with a small amount of diethyl ether to give an orange solid, which was intermediate XI.The yield was 65%.
References:
CN109180702,2019,A Location in patent:Paragraph 0152; 0156; 0158; 0166-0169
2615-25-0
281 suppliers
$5.00/1g
![(1R,4R)-[4-(3-Nitro-pyridin-2-ylaMino)-cyclohexyl]-carbaMic acid tert-butyl ester](/CAS/20120318/GIF/CB62574899.gif)
1289386-01-1
7 suppliers
inquiry

24424-99-5
864 suppliers
$13.50/25G
![(1R,4R)-[4-(3-Nitro-pyridin-2-ylaMino)-cyclohexyl]-carbaMic acid tert-butyl ester](/CAS/20120318/GIF/CB62574899.gif)
1289386-01-1
7 suppliers
inquiry