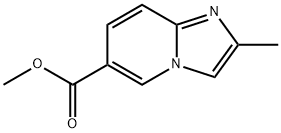
IMidazo[1,2-a]pyridine-6-carboxylic acid, 2-Methyl-, Methyl ester synthesis
- Product Name:IMidazo[1,2-a]pyridine-6-carboxylic acid, 2-Methyl-, Methyl ester
- CAS Number:129912-28-3
- Molecular formula:C10H10N2O2
- Molecular Weight:190.2
Yield:129912-28-3 54%
Reaction Conditions:
in ethanol; for 24 h;Reflux;
Steps:
1.1 [Step 1]
Preparation of methyl 2-methylimidazo[1,2-a]pyridine-6-carboxylate
[Step 1]
Preparation of methyl 2-methylimidazo[1,2-a]pyridine-6-carboxylate
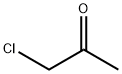
6-aminonicotinic acid methylester (500 mg, 3.29 mmol) and chloroacetone (1.83 g, 19.74 mmol) were dissolved in ethanol (15 mL), followed by refluxing and stirring for 24 hours.
After removing a solvent, the resultant was diluted with water (15 mL), basified by sodium hydrogen carbonate saturated aqueous solution and then extracted three times with methylene chloride (MC, 10 mL).
The organic layer was collected, dried over anhydrous magnesium sulfate, concentrated under reduce pressure, and purified by column chromatography (MC: MeOH=40:1), thereby obtaining methyl 2-methylimidazo[1,2-a]pyridine-6-carboxylate (340 mg, 54%).
1H NMR (600 MHz, chloroform-d1) d=8.80 (s, 1H), 7.65 (d, J=9.6 Hz, 1H), 7.45 (d, J=9.6 Hz, 1H), 7.38 (s, 1H) 113, 3.91 (s, 3H), 2.45 (s, 3H)
References:
US2013/165652,2013,A1 Location in patent:Paragraph 0068; 0069
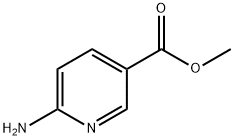
36052-24-1
251 suppliers
$10.00/1g
598-31-2
109 suppliers
$60.00/50mg
![IMidazo[1,2-a]pyridine-6-carboxylic acid, 2-Methyl-, Methyl ester](/CAS/GIF/129912-28-3.gif)
129912-28-3
21 suppliers
inquiry