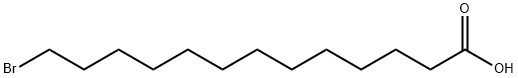
13-bromotridecanoic acid synthesis
- Product Name:13-bromotridecanoic acid
- CAS Number:15462-16-5
- Molecular formula:C13H25BrO2
- Molecular Weight:293.24

116754-58-6
74 suppliers
$46.00/100mg

15462-16-5
11 suppliers
inquiry
Yield: 99%
Reaction Conditions:
with 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;[bis(acetoxy)iodo]benzene in water;acetonitrile at 0 - 20; for 4 h;Inert atmosphere;
Steps:
4.2.23. 13-Bromotridecanoic acid (45)
To a solution of 44 (0.45 g, 1.6 mmol) in CH3CN/H2O (2:1, 6.6 mL)were added TEMPO (51 mg, 0.33 mmol) and [bis(acetoxy)iodo]benzene(1.3 g, 4.1 mmol) at 0 °C. After stirring for 4 h at rt, the completionof the reaction was confirmed by TLC (1:1 n-hexane/EtOAc). The reactionmixture was diluted with EtOAc and washed with H2O and brine.The organic layer was subsequently dried over Na2SO4, filtered, andconcentrated. The resulting residue was purified by silica gel columnchromatography (10:1 n-hexane/EtOAc) to give 45 (0.47 g, 99%) aswhite amorphous solid. 1H NMR (500 MHz, CDCl3) δ 3.41 (t, 2H,Jvic=7.0 Hz, CH2Br), 2.35 (t, 2H, Jvic=7.5 Hz, COCH2), 1.881.82 (m,2H, CH2CH2Br), 1.65-1.60 (m, 2H, COCH2CH2), 1.41 (near t, 2H,CH2CH2CH2Br), 1.34-1.27 (m,14H, -CH2-); 13C NMR (125 MHz, CDCl3)δ 179.2, 34.1, 34.0, 32.9, 29.5, 29.5, 29.4, 29.4, 29.1, 28.8, 28.2, 24.7.HRMS (ESI) m/z: found [M-H]- 291.0969, C13H24O2Br calcd for[M-H]- 291.0965.
References:
Takato, Koichi;Kurita, Motoki;Yagami, Nahoko;Tanaka, Hide-Nori;Ando, Hiromune;Imamura, Akihiro;Ishida, Hideharu [Carbohydrate Research,2019,vol. 483,art. no. 107748]

13362-52-2
105 suppliers
$11.00/250mg

15462-16-5
11 suppliers
inquiry
505-52-2
239 suppliers
$6.00/1g

15462-16-5
11 suppliers
inquiry
503154-44-7
0 suppliers
inquiry

15462-16-5
11 suppliers
inquiry

