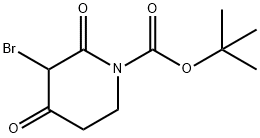
3-BroMo-2,4-dioxo-piperidine-1-carboxylic acid tert-butyl ester synthesis
- Product Name:3-BroMo-2,4-dioxo-piperidine-1-carboxylic acid tert-butyl ester
- CAS Number:1312412-87-5
- Molecular formula:C10H14BrNO4
- Molecular Weight:292.13
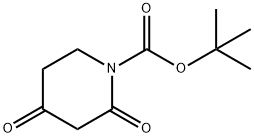
845267-78-9

1312412-87-5
Tert-butyl 2,4-dioxopiperidine-1-carboxylate (1 g, 4.69 mmol) was used as starting material and dissolved in anhydrous carbon tetrachloride (10 mL) with stirring. N-bromosuccinimide (0.83 g, 4.69 mmol) was added slowly at 10 °C. The reaction temperature was maintained in the range of 10-15 °C with continuous stirring for 2 hours. After completion of the reaction, the solvent was evaporated under reduced pressure. Water (10 mL) was added for dilution and the target product 3-bromo-2,4-dioxopiperidine-1-carboxylic acid tert-butyl ester was subsequently extracted with ethyl acetate (2 x 30 mL). The organic layers were combined, dried with anhydrous sodium sulfate and concentrated to obtain the crude product. Purification of the crude product by fast column chromatography resulted in the title compound in 99% yield (1.4 g, off-white solid). The structure of the product was confirmed by 1H NMR (400 MHz, DMSO-d6): δ 5.50 (s, 1H), 3.74-3.71 (m, 2H), 2.69-2.66 (m, 2H), 1.46 (s, 9H).The LCMS analysis (Method A) showed: 193.8 (M-Boc + H), retention time 2.93 min, purity 81.51% (max).

845267-78-9
173 suppliers
$10.00/1g

1312412-87-5
29 suppliers
$80.00/100mg
Yield:1312412-87-5 99%
Reaction Conditions:
with N-Bromosuccinimide in tetrachloromethane at 10 - 15; for 2 h;
Steps:
25.1 Step 1: tert-butyl 3-bromo-2,4-dioxopiperidine-1-carboxylate
To a stirred solution of tert-butyl 2,4-dioxopiperidine-1-carboxylate (1 g, 4.69 mmol) in dry CCl4 (10 mL), N-bromosuccinimide (0.83 g, 4.69 mmol) was added at 10 °C. The reaction mixture was stirred at 10-15 °C for 2 h. It was then evaporated under reduced pressure. Water (10 mL) was added and the desired product was extracted with EtOAc (2 x 30 mL). The combined organic layer was dried over Na2SO4 and concentrated. The resulting crude product was purified by flash column chromatography, affording the title product. Yield: 99% (1.4 g, off white solid). 1H NMR (400 MHz, DMSO-d6): δ 5.50 (s, 1H), 3.74-3.71 (m, 2H), 2.69-2.66 (m, 2H), 1.46 (s, 9H). LCMS: (Method A) 193.8 (M-Boc+H), Rt. 2.93min, 81 .51 % (Max).
References:
WO2016/30443,2016,A1 Location in patent:Page/Page column 96
3303-84-2
371 suppliers
$6.00/5g

1312412-87-5
29 suppliers
$80.00/100mg

24424-99-5
869 suppliers
$13.50/25G

1312412-87-5
29 suppliers
$80.00/100mg