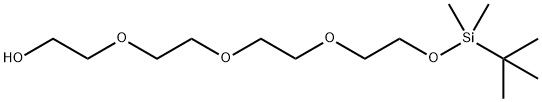
2-[2-[2-[2-[(tert-Butyldimethylsilanyl)oxy]ethoxy]ethoxy]ethoxy]ethanol synthesis
- Product Name:2-[2-[2-[2-[(tert-Butyldimethylsilanyl)oxy]ethoxy]ethoxy]ethoxy]ethanol
- CAS Number:134179-40-1
- Molecular formula:C14H32O5Si
- Molecular Weight:308.49

18162-48-6
679 suppliers
$9.00/5g
![2-[2-[2-[2-[(tert-Butyldimethylsilanyl)oxy]ethoxy]ethoxy]ethoxy]ethanol](/CAS/20180703/GIF/134179-40-1.gif)
134179-40-1
41 suppliers
$90.00/100mg
Yield: 61%
Reaction Conditions:
Stage #1:Tetraethylene glycol with 1H-imidazole in N,N-dimethyl-formamide at 0; for 0.5 h;
Stage #2:tert-butyldimethylsilyl chloride in N,N-dimethyl-formamide at 0; for 2 h;
Steps:
Preparation of tert-butyl-dimethyl-silanyloxy tetraethyleneglycol (Compound 1):Compound 1A solution of imidazole (7.0 grams, 102.8 mmol) and tetraethyleneglycol (30.0 grams, 154.4 mmol) in dry DMF (70 ml) was cooled to 0 °C and stirred for 30 minutes under argon atmosphere. Tertbutyldimethylsilyl chloride (15.5 g, 102.8 mmol) in dry DMF (50 ml) was added dropwise to the solution, and stirring continued for two additional hours at 0 °C. Thereafter the reaction mixture was allowed to warm to room temperature, water (900 ml) was added and the resulting solution was extracted with ethyl acetate (4 portions of 400 ml). The combined organic extracts were washed with brine and the solvent was evaporation under reduced pressure to give a crude product. The crude product was purified by flash chromatography on silica using ethyl acetate as eluent to give Compound 1 (19.2 grams, 61 % yield) as a light yellow oil.
References:
RAMOT AT TEL AVIV UNIVERSITY LTD.;THE BRIGHAM AND WOMEN'S HOSPITAL, INC. WO2006/77597, 2006, A2 Location in patent:Page/Page column 66
![Bis[2-(2-hydroxyethoxy)ethyl] ether](/CAS/GIF/112-60-7.gif)
112-60-7
372 suppliers
$7.00/25g

18162-48-6
679 suppliers
$9.00/5g
![2-[2-[2-[2-[(tert-Butyldimethylsilanyl)oxy]ethoxy]ethoxy]ethoxy]ethanol](/CAS/20180703/GIF/134179-40-1.gif)
134179-40-1
41 suppliers
$90.00/100mg