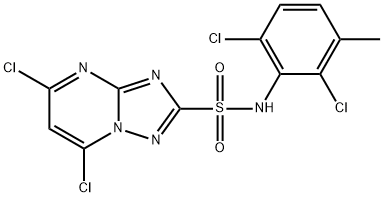
N-(2,6-Dichloro-3-methylphenyl)-5,7-dichloro-1,2,4-triazolo[1,5-a]pyrimidine-2-sulfonamide synthesis
- Product Name:N-(2,6-Dichloro-3-methylphenyl)-5,7-dichloro-1,2,4-triazolo[1,5-a]pyrimidine-2-sulfonamide
- CAS Number:134892-32-3
- Molecular formula:C12H7Cl4N5O2S
- Molecular Weight:427.09
Yield:134892-32-3 73%
Reaction Conditions:
with trichlorophosphate in acetonitrile;
Steps:
3 Preparation of 5,7-Dichloro-N-(2,6-dichloro-3-methylphenyl)-1,2,4-triazolo[1,5-a]pyrimidine-2-sulfonamide STR8
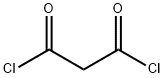
EXAMPLE 3 Preparation of 5,7-Dichloro-N-(2,6-dichloro-3-methylphenyl)-1,2,4-triazolo[1,5-a]pyrimidine-2-sulfonamide STR8 To a stirred solution of 6.44 g (0.02 moles) of N-(3-(((2,6-dichloro-3-methylphenyl)amino)sulfonyl)-1H-1,2,4-triazol-5-yl)amine in 100 mL of dry acetonitrile was added 2.82 g (0.02 moles) of freshly distilled malonyl chloride. The mixture was stirred at room temperature overnight after which time some starting material remained. Additional malonyl chloride (0.56 g) was added and stirring was continued for 4 hrs. The reaction mixture was concentrated to dryness and the residue was slurried with POCl3 (122 g; 0.8 moles) and heated at 90° C. for 9 hrs. The reaction mixture was cooled and concentrated under reduced pressure and the residue was poured into ice-water. The resulting precipitate was filtered and dried to give 8.35 g of product having a purity of 75 percent (73 percent yield).
References:
US5006656,1991,A
![[1,2,4]Triazolo[1,5-a]pyrimidine-2-sulfonamide, N-(2,6-dichloro-3-methylphenyl)-1,5-dihydro-7-hydroxy-5-oxo-](/CAS/20210305/GIF/134892-30-1.gif)
134892-30-1
0 suppliers
inquiry
![N-(2,6-Dichloro-3-methylphenyl)-5,7-dichloro-1,2,4-triazolo[1,5-a]pyrimidine-2-sulfonamide](/CAS/GIF/134892-32-3.gif)
134892-32-3
10 suppliers
inquiry