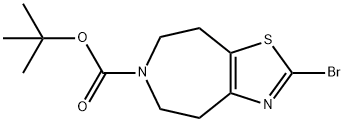
tert-butyl 2-broMo-4,5,7,8-tetrahydrothiazolo[5,4-d]azepine-6-carboxylate synthesis
- Product Name:tert-butyl 2-broMo-4,5,7,8-tetrahydrothiazolo[5,4-d]azepine-6-carboxylate
- CAS Number:1352925-59-7
- Molecular formula:C12H17BrN2O2S
- Molecular Weight:333.24
![tert-butyl 2-amino-4,5,7,8-tetrahydrothiazolo[5,4-d]azepine-6-carboxylate](/CAS/GIF/1440954-94-8.gif)
1440954-94-8
10 suppliers
inquiry
![tert-butyl 2-broMo-4,5,7,8-tetrahydrothiazolo[5,4-d]azepine-6-carboxylate](/CAS/20150408/GIF/1352925-59-7.gif)
1352925-59-7
11 suppliers
inquiry
Yield:1352925-59-7 51%
Reaction Conditions:
with tert.-butylnitrite;copper(ll) bromide in acetonitrile at 60; for 1 h;
Steps:
Step 2: 2-Bromo-4,5,7,8-tetrahydro-thiazolo[4,5-d]azepine-6-carboxylic acid tert-butyl ester
tert-Butyl-nitrite was added to 3.31 g (15 mmol) copper-(II)-bromide in 100 mL ACN and stirred at RT for 10 min. The reaction mixture was stirred at 60° C. and 2.00 g (7.43 mmol) 2-amino-4,5,7,8-tetrahydro-thiazolo[4,5-d]azepine-6-carboxylic acid tert-butyl ester was added and the reaction was stirred for 1 h at 60° C. The reaction was concentrated to dryness and ethylacetate and water were added to the residue. After filtration the organic layer was separated, dried and concentrated to dryness. The residue was purified by chromatography. [0098] Yield: 1.26 g (51% of theory) [0099] ESI-MS: m/z=333 (M+H)+
References:
US2013/261105,2013,A1 Location in patent:Paragraph 0096-0099

24424-99-5
857 suppliers
$13.50/25G
![tert-butyl 2-broMo-4,5,7,8-tetrahydrothiazolo[5,4-d]azepine-6-carboxylate](/CAS/20150408/GIF/1352925-59-7.gif)
1352925-59-7
11 suppliers
inquiry