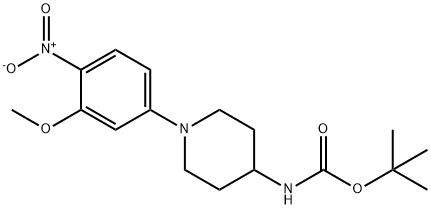
[1-(3-Methoxy-4-nitro-phenyl)-piperidin-4-yl]-carbaMic acid tert-butyl ester synthesis
- Product Name:[1-(3-Methoxy-4-nitro-phenyl)-piperidin-4-yl]-carbaMic acid tert-butyl ester
- CAS Number:1356962-89-4
- Molecular formula:C17H25N3O5
- Molecular Weight:351.4
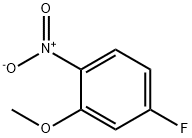
448-19-1
304 suppliers
$5.00/1g
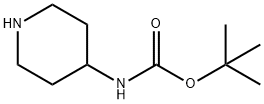
73874-95-0
456 suppliers
$10.00/250mg
![[1-(3-Methoxy-4-nitro-phenyl)-piperidin-4-yl]-carbaMic acid tert-butyl ester](/CAS/20150408/GIF/1356962-89-4.gif)
1356962-89-4
14 suppliers
inquiry
Yield: 71.2%
Reaction Conditions:
with potassium carbonate in acetonitrile for 3 h;Inert atmosphere;
Steps:
1.1 Step 1 Synthesis of Compound 3
Add compound 1 to a 50 mL single-mouth flask equipped with magnetic stirring(1.71 g, 10 mmol) and acetonitrile(30 mL), stirred and dissolved, and added compound 2 (2.6 g, 13 mmol)And potassium carbonate (2.07g, 15mmol),The reaction mixture was heated to 70 ° C under a nitrogen atmosphere and stirred for 3 hours while stirring.Cool to room temperature, distill off the solvent under reduced pressure and add water (60 mL).A large amount of yellow solid was precipitated, filtered, washed with water (10 mL).Drying the yellow solid2.5g,The yield was 71.2%.
References:
Shenzhen Tajirui Bio-pharmaceutical Co., Ltd.;Wang Yihan;Li Huanyin CN109851638, 2019, A Location in patent:Paragraph 0215-0220