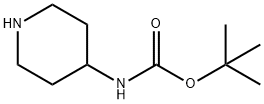
4-N-BOC-Aminopiperidine synthesis
- Product Name:4-N-BOC-Aminopiperidine
- CAS Number:73874-95-0
- Molecular formula:C10H20N2O2
- Molecular Weight:200.28
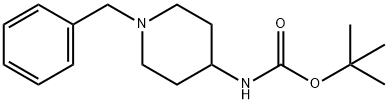
73889-19-7

73874-95-0
General procedure for the synthesis of 4-tert-butoxycarbonylaminopiperidine from tert-butyl (1-benzylpiperidin-4-yl)carbamate: N-butyl-1-benzylpiperidin-4-ylcarbamate (3.80 g, 13.1 mmol) was dissolved in 26 mL of methanol in a 100 mL reaction vessel. A catalytic amount of 10% palladium/carbon (Pd/C) was added. The reaction was stirred under hydrogen atmosphere for 12 hours. Upon completion of the reaction, the palladium/carbon catalyst was removed by filtration through a diatomaceous earth pad and the filtrate was concentrated under reduced pressure to remove the solvent. The resulting residue was purified by silica gel column chromatography to afford 4-tert-butoxycarbonylaminopiperidine (2.64 g, 99% yield). The product was confirmed by 1H-NMR (200 MHz, CD3OD): δ 1.36 (s, 9H), 1.84-2.36 (m, 4H), 2.74 (m, 2H), 3.42 (m, 2H), 3.60 (br, 1H).LC/MS analysis showed the [M+H]+ peak as 201.

73889-19-7
119 suppliers
$25.00/5g

73874-95-0
441 suppliers
$10.00/250mg
Yield:73874-95-0 99%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in methanol; for 12 h;
Steps:
6; 1-ii; 7; 2-ii; 9; 4-ii
J-Butyl-l-benzylpiperidin-4-ylcarbamate (3.80 g, 13.1 mmol) obtained in Preparation Example 1-i was dissolved in 26 ml of methanol in a 100 ml vessel, and a catalytic quantity of 10% active palladium/carbon was added thereto, and the resulting mixture was reacted under a hydrogen atmosphere for 12 hrs. After the completion of reaction, the reaction mixture was filtered through a cellite pad to remove the active palladium/carbon and the solvent was removed under reduced pressure therefrom. Then, the residue was subjected to a silica gel column chromatography to obtain 2.64 g of the title compound (yield 99%).1H-NMR (200 MHz, CD3OD) δ 1.36 (s, 9H), 1.84-2.36 (m, 4H), 2.74 (m, 2H), 3.42 (m, 2H), 3.60 (br, IH). LC/MS (M+H): 201; r-Butyl-l-benzylpiperidin-4-ylcarbamate (3.80 g, 13.1 mmol) obtained in Preparation Example 2-i was dissolved in methanol (26 ml) in a 100 ml vessel, and a catalytic quantity of 10% active palladium/carbon was added thereto. The resulting mixture was reacted under a hydrogen atmosphere for 12 hrs. After the completion of reaction, the reaction mixture was filtered through a cellite pad to remove the active palladium/carbon and the solvent was removed under reduced pressure. Then, the mixture was subjected to a silica gel column chromatography to obtain 2.64 g of the title compound (yield 99%).1H-NMR (200 MHz, CD3OD) δ 1.36 (s, 9H), 1.84-2.36 (m, 4H), 2.74 (m, 2H), 3.42 (m, 2H), 3.60 (br, IH). LC/MS (M+H): 201; /-Butyl- l-benzylpiperidin-4-ylcarbamate (3.801 g, 13.1 mmol) obtained in Preparation Example 4-i was dissolved in 26 ml of methanol in a100 ml vessel, and a catalytic quantity of 10% active palladium/carbon was added thereto, and the resulting mixture was reacted under a hydrogen5 atmosphere for 12 hrs. After the completion of reaction, the reaction mixture was filtered through a cellite pad to remove the active palladium/carbon catalyst and the solvent was removed under reduced pressure therefrom. Then, the residue thus obtained was subjected to a silica gel column chromatography to obtain 2.64 g of the title compound (yieldLO 99%).1H-NMR (200 MHz, CD3OD) δ 1.36 (s, 9H), 1.84-2.36 (m, 4H), 2.74 (m, 2H), 3.42 (m, 2H), 3.60 (br, IH).LC/MS (M+H): 201.
References:
WO2008/54154,2008,A1 Location in patent:Page/Page column 33; 34; 76; 77; 162-164
50541-93-0
353 suppliers
$6.00/5g

24424-99-5
871 suppliers
$13.50/25G

73874-95-0
441 suppliers
$10.00/250mg

50541-93-0
353 suppliers
$6.00/5g

73874-95-0
441 suppliers
$10.00/250mg
159874-20-1
47 suppliers
$250.00/1g

73874-95-0
441 suppliers
$10.00/250mg
60211-57-6
241 suppliers
$8.00/1g

73874-95-0
441 suppliers
$10.00/250mg