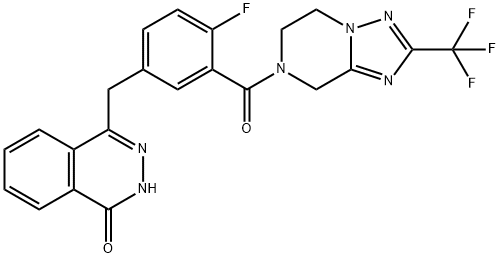
Fluzoparib synthesis
- Product Name:Fluzoparib
- CAS Number:1358715-18-0
- Molecular formula:C22H16F4N6O2
- Molecular Weight:472.4

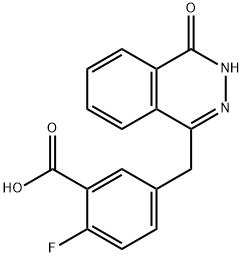
763114-26-7
301 suppliers
$6.00/250mg
![2-(TRIFLUOROMETHYL)-5,6,7,8-TETRAHYDRO-[1,2,4]TRIAZOLO[1,5-A]PYRAZINE](/CAS/GIF/681249-57-0.gif)
681249-57-0
79 suppliers
$96.00/100mg

1358715-18-0
39 suppliers
inquiry
Yield: 16.4%
Reaction Conditions:
with O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate;N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide for 12 h;Inert atmosphere;
Steps:
19 4-[[4-fluoro-3-[2-(trifluoromethyl)-6,8-dihydro-5H-[1,2,4]triazolo[1,5-a]pyrazine-7-carbonyl]phenyl]methyl]-2H-phthalazin-1-one
2-Fluoro-5-[(4-oxo-3H-phthalazin-1-yl)methyl]benzoic acid 1a (780 mg, 2.65 mmol) was dissolved in 15 mL of N, N-dimethylformamide, followed by addition of O-(1-benzotriazolyl)-N, N, N', N'-tetramethyluronium hexafluorophosphate (1.80 g, 4.77 mmol), 2-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[1,5-a]pyrazine 19a (560 mg, 2.92 mmol, prepared according to a known method disclosed by 'patent application ') and N, N-diisopropylethylamine (1.4 mL, 7.95 mmol).
After stirring for 12 hours, the reaction mixture was concentrated under reduced pressure, added with 30 mL of H2O, extracted with ethyl acetate (30 mL*3).
The organic phase was combined, washed with saturated sodium chloride solution (20 mL), dried over anhydrous sodium sulfate and filtered.
The filtrate was concentrated under reduced pressure and the resulting residue was purified by thin layer chromatography with elution system A to obtain 4-[[4-fluoro-3-[2-(trifluoromethyl)-6,8-dihydro-5H-[1,2,4]triazolo[1,5-a]pyrazine-7-carbonyl]phenyl]methyl]-2H-phthalazin-1-one 19 (205 mg, yield 16.4%) as a light yellow solid.
MS m/z (ESI): 473.1 [M+1]
1H NMR (400 MHz, CDCl3): δ 10.67 (br. s, 1H), 8.48 (s, 1H), 7.77 (m, 3H), 7.42 (m, 2H), 7.11 (t, 1H), 5.10 (s, 1H), 4.75 (s ,1H),4.39 (s, 2H), 4.32 (d, 3H), 3.88 (s, 1H)
References:
Jiangsu Hansoh Pharmaceutical Co., Ltd.;TANG, Pengcho;LI, Xin;LI, Xiangqin;CHEN, Yang;WANG, Bin;ZHU, Zhe EP2604610, 2013, A1 Location in patent:Paragraph 0134; 0135

763114-26-7
301 suppliers
$6.00/250mg
![2-(TRIFLUOROMETHYL)-[1,2,4]TRIAZOLO[1,5-A]PYRAZINE](/CAS/GIF/681249-56-9.gif)
681249-56-9
64 suppliers
inquiry

1358715-18-0
39 suppliers
inquiry
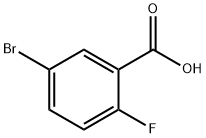
146328-85-0
290 suppliers
$6.00/1g

1358715-18-0
39 suppliers
inquiry