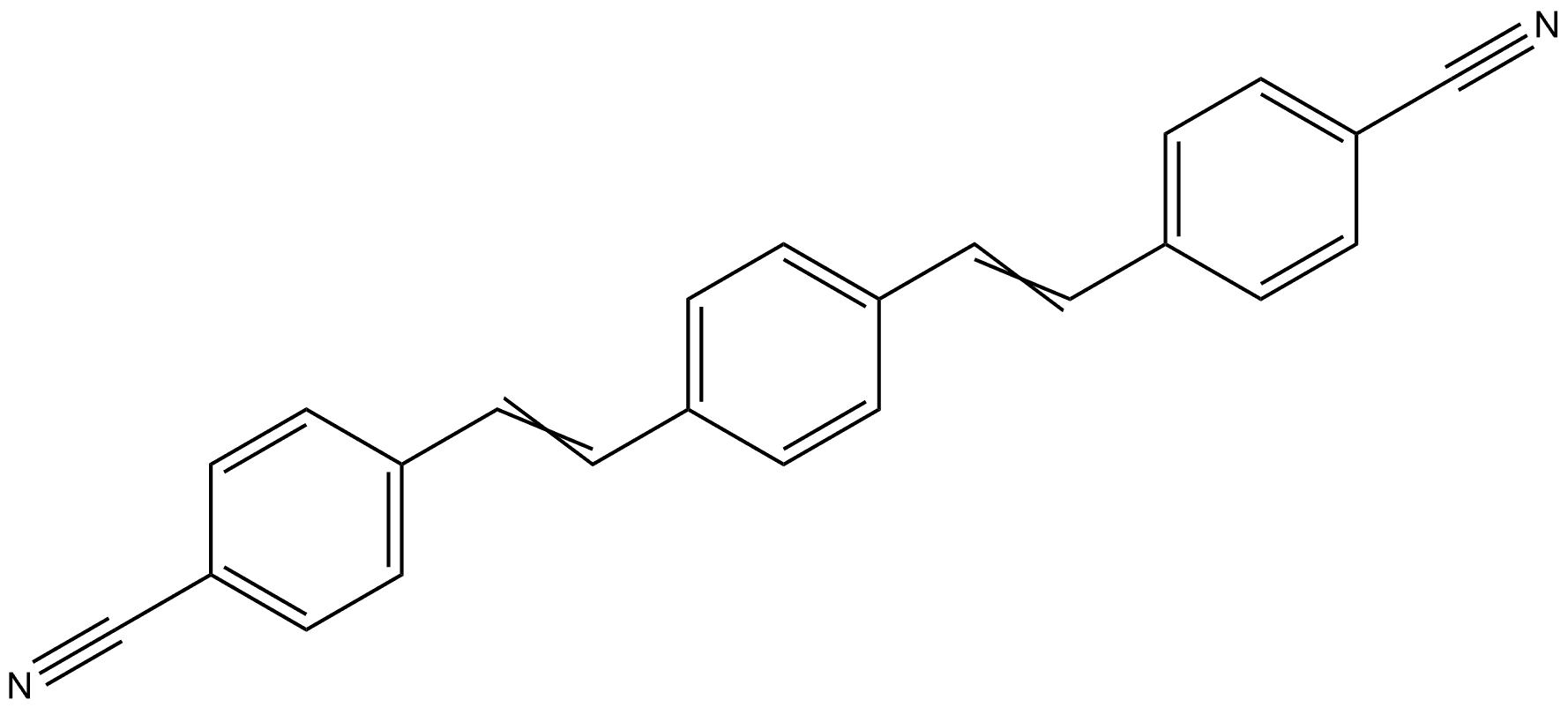
Benzonitrile, 4,4'-[(2E)-1,4-phenylenedi-2,1-ethenediyl]bis- synthesis
- Product Name:Benzonitrile, 4,4'-[(2E)-1,4-phenylenedi-2,1-ethenediyl]bis-
- CAS Number:136384-76-4
- Molecular formula:C24H16N2
- Molecular Weight:332.4
Yield:136384-76-4 90%
Reaction Conditions:
with triphenyl phosphite;triethylamine;bis(dibenzylideneacetone)-palladium(0) in N,N-dimethyl-formamide at 110; for 48 h;Sealed tube;Inert atmosphere;Heck Reaction;
Steps:
2.4. Synthesis of oligo(phenylene vinylene)s 1a-i and 2a-g
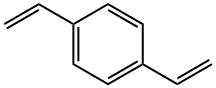
General procedure: according to the Mizoroki-Heck reaction (Scheme 1). First; by thecoupling between 4-substituted styrenes and dibromo or diiodobenzenes(Method A, Scheme 1), and second; the reaction between4-substituted bromo or iodobenzenes with 1,4-divinylbenzene(Method B, Scheme 1).The general procedure is as follows: 2.05 equivalents of thecorresponding styrene and 1 equivalent of 1,4-dibromo or 1,4-diiodobenzene (Method A, to OPVs 1a-d and 2a-f) or 2.05 equivalentsof the appropriate aryl halide and 1 equivalent of 1,4-divinylbenzene (Method B, to OPVs 1e-h), were placed in a 10 mLheadspace crimp vial equipped with a magnetic stir bar. Then, forboth methods, it was added 0.01 equivalents (1 mol%) of Pd(dba)2,0.1 equivalents (10 mol%) of P(OPh)3 and 5 equivalents of TEA. Thevial was sealed with a PTFE/silicone septum with aluminum capand purged-saturated with N2, then 2 mL of solvent was injected(dry DMF for OPVs-series 1 and 1,4-dioxane for OPVs-series 2,Scheme 1) and the system was purged once again with N2. Thereaction mixture was vigorously stirred at 110 C during 48 h forOPVs-series 1 and 24 h for OPVs-series 2. After finished, highlyfluorescent solid products were obtained.
References:
Estrada, Sandra E.;Ochoa-Puentes, Cristian;Sierra, Cesar A. [Journal of Molecular Structure,2017,vol. 1133,p. 448 - 457]
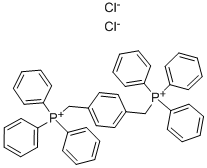
4546-04-7
124 suppliers
$60.00/100mg
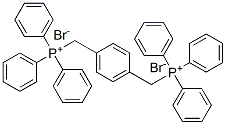
105-07-7
396 suppliers
$5.00/5g
![Benzonitrile, 4,4'-[(2E)-1,4-phenylenedi-2,1-ethenediyl]bis-](/CAS/20210305/GIF/136384-76-4.gif)
136384-76-4
1 suppliers
inquiry