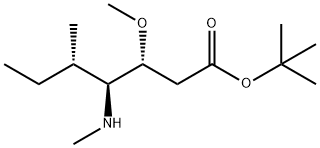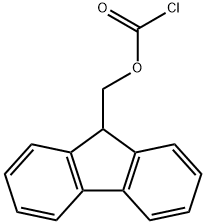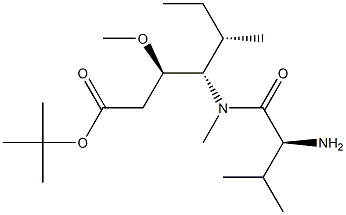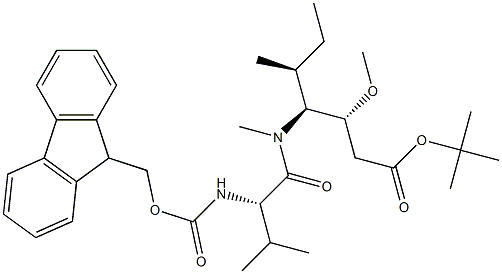
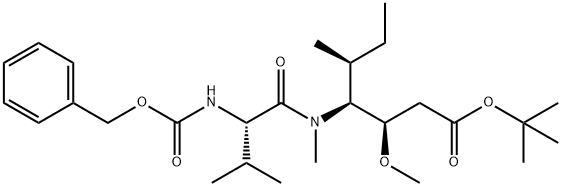
tert-butyl (3R,4S,5S)-4-((S)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-N,3-dimethylbutanamido)-3-methoxy-5-methylheptanoate synthesis
- Product Name:tert-butyl (3R,4S,5S)-4-((S)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-N,3-dimethylbutanamido)-3-methoxy-5-methylheptanoate
- CAS Number:1375415-93-2
- Molecular formula:C34H48N2O6
- Molecular Weight:580.75
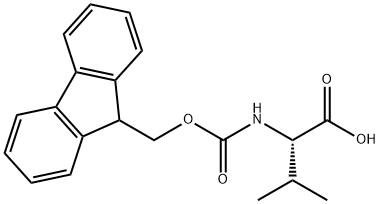
68858-20-8
544 suppliers
$8.00/1g
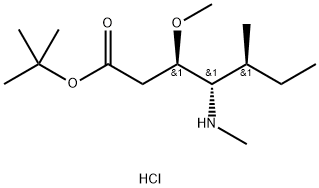
120205-48-3
124 suppliers
$86.00/100mg

1375415-93-2
5 suppliers
inquiry
Yield:1375415-93-2 486 mg
Reaction Conditions:
with 2-chloro-4,6-dimethoxy-1 ,3,5-triazine at 20;
Steps:
1.1.1.c Step c: Synthesis of Fmoc-L-Val-Dil-OBut.
The reactant Dil-OBut.HCl (500 mg, 1.7 mmol) andFmoc-L-Val-OH (780 mg, 2.3 mmol) dissolved in 10 mLIn 2-Me-THF, CDMT (403.5 mg, 2.3 mmol) and NMM (256.6 mg, 2.55 mmol) were sequentially added, and the mixture was stirred overnight at room temperature.. After the reaction was completed, ethyl acetate was added for extraction three times.The organic phases were combined and washed once with saturated NaCl.Dry overnight with anhydrous Na2SO4. Column chromatography separation,The separation conditions were petroleum ether: ethyl acetate = 6:1,The reaction product was obtained 486 mg.
References:
CN109200291,2019,A Location in patent:Paragraph 0051; 0057
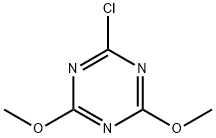
3140-73-6
512 suppliers
$9.00/25g

68858-20-8
544 suppliers
$8.00/1g

1375415-93-2
5 suppliers
inquiry
120205-52-9
5 suppliers
inquiry

1375415-93-2
5 suppliers
inquiry