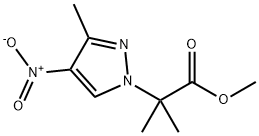
methyl 2-methyl-2-(3-methyl-4-nitro-1H-pyrazol-1-yl)propanoate synthesis
- Product Name:methyl 2-methyl-2-(3-methyl-4-nitro-1H-pyrazol-1-yl)propanoate
- CAS Number:1393102-03-8
- Molecular formula:C9H13N3O4
- Molecular Weight:227.2172
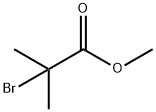
23426-63-3
250 suppliers
$9.00/1g
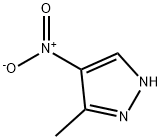
5334-39-4
162 suppliers
$27.00/25g

1393102-03-8
16 suppliers
inquiry
Yield:1393102-03-8 97.47%
Reaction Conditions:
Stage #1: 3-methyl-4-nitro-1H-pyrazolewith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0 - 25; for 2.5 h;Inert atmosphere;
Stage #2: 2-bromo-2-methylpropionic acid methyl ester in N,N-dimethyl-formamide;mineral oil at 0 - 25; for 16 h;Inert atmosphere;
Steps:
A-1; B-1; C-9 Methyl 2-methyl-2-(3-methyl-4-nitro-pyrazol-1-yl)propanoate
To a solution of 3-methyi-4- mtro-lH-pyrazole (40 g, 314.71 mmol) in DMF (700 mL) was added NaH (18.88 g, 472.06 mmol, 60% purity) at 0 °C over a period of 30 min under N2. The reaction was then stirred at 25 °C for 2 h followed by the addition of methyl 2-bromo-2-methylpropanoate (85.46 g, 472.06 mmol, 61.04 mL) dropwise at 0°C. The reaction mixture was warmed to 25 °C and stirred at 25 °C for another 16 h. TLC (petroleum ether/ethyl acetate = 5: 1) showed the starting material was consumed completely. The reaction was quenched by ice water slowly and then extracted with EtOAc (3 χ 700 mL). The combined organic phase was washed with brine (3 χ 200 mL), dried over anhydrous Na2S(, filtered and concentrated in vacuo. The residue was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 30: 1- 15: 1), to yield the desired product as a light yellow solid. H NMR (400 MHz, C DC M: 5 8.29 (s, 1 H), 3.72 is. 1 1 1). 2.51 (s, I H), 1 .84 (s, 6 H).
References:
WO2017/87905,2017,A1 Location in patent:Page/Page column 104