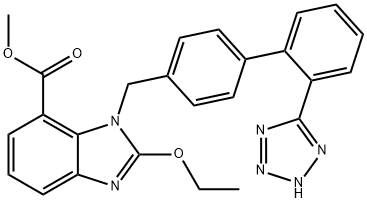
Ethyl-2-Ethoxy-1-[[(2'-(1h-Tetrazol-5-Yl)Biphenyl-4-Yl)Methyl]Benzimidazole]-7-Carboxylate synthesis
- Product Name:Ethyl-2-Ethoxy-1-[[(2'-(1h-Tetrazol-5-Yl)Biphenyl-4-Yl)Methyl]Benzimidazole]-7-Carboxylate
- CAS Number:139481-69-9
- Molecular formula:C25H22N6O3
- Molecular Weight:454.48
![Methyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-1H-benzimidazole-7-carboxylate](/CAS/GIF/139481-44-0.gif)
139481-44-0
![Ethyl-2-Ethoxy-1-[[(2'-(1h-Tetrazol-5-Yl)Biphenyl-4-Yl)Methyl]Benzimidazole]-7-Carboxylate](/CAS/GIF/139481-69-9.gif)
139481-69-9
1. Methyl 2-ethoxy-1-[(2'-cyanobiphenyl-4-yl)methyl]-1H-benzimidazole-7-carboxylate (310 g) and toluene (2.48 L) were added to a reactor, followed by tributyltin chloride (737 g), sodium azide (146 g) and tetrabutylammonium bromide (31 g). 2. The reaction mixture was slowly heated to 110 °C and maintained at 110-115 °C for 24 h, during which the progress of the reaction was monitored by TLC. 3. After completion of the reaction, the reaction mixture was cooled to 15 °C and methanol (3.1 L), water (2.17 L) and acetic acid (930 g) were added sequentially. 4. The mixture was stirred at 15-20 °C for 1 h to promote product separation. 5. After addition of toluene (1.24 L), the reaction mixture was filtered at 15-20 °C. 6. the filter cake was washed thoroughly with water (0.93 L) until the pH of the wash solution reached 5.5-7.0 to completely remove the acid. 7. The filter cake was washed again with toluene (0.62 L) and dried by suction filtration. 8. The product was air dried at 50-55 °C to give methyl 2-ethoxy-1-[[(2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl]benzimidazole]-7-carboxylate. Yield: 290 g (85%).
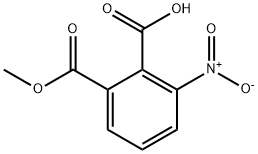
21606-04-2
147 suppliers
$7.00/1g
![Ethyl-2-Ethoxy-1-[[(2'-(1h-Tetrazol-5-Yl)Biphenyl-4-Yl)Methyl]Benzimidazole]-7-Carboxylate](/CAS/GIF/139481-69-9.gif)
139481-69-9
149 suppliers
$8.00/250mg
Yield:-
Steps:
Multi-step reaction with 8 steps
1: SOCl2, DMF / toluene / 0.5 h / Heating
2: NaN3 / acetone; H2O / 1 h
3: 1.5 h / Heating
4: 85 percent / K2CO3 / acetonitrile / 4 h / Heating
5: 77 percent / 30percent ethanolic HCl / ethyl acetate / 1 h / Ambient temperature
6: 1.) FeCl3*6H2O, activated carbon, 2.) hydrazine monohydrate / 1.) MeOH, THF, reflux, 30 min, 2.) MeOH, THF, reflux, 14 h
7: 91 percent / acetic acid / 0.67 h / 80 - 90 °C
8: 100 percent / trimethyltin azide / toluene / 29 h / Heating
References:
Kubo, Keiji;Kohara, Yasuhisa;Imamiya, Eiko;Sugiura, Yoshihiro;Inada, Yoshiyuki;et al. [Journal of Medicinal Chemistry,1993,vol. 36,# 15,p. 2182 - 2195]
![Methyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-1H-benzimidazole-7-carboxylate](/CAS/GIF/139481-44-0.gif)
139481-44-0
331 suppliers
$9.00/5g
![Ethyl-2-Ethoxy-1-[[(2'-(1h-Tetrazol-5-Yl)Biphenyl-4-Yl)Methyl]Benzimidazole]-7-Carboxylate](/CAS/GIF/139481-69-9.gif)
139481-69-9
149 suppliers
$8.00/250mg
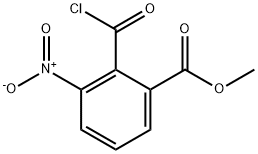
73833-13-3
7 suppliers
inquiry
![Ethyl-2-Ethoxy-1-[[(2'-(1h-Tetrazol-5-Yl)Biphenyl-4-Yl)Methyl]Benzimidazole]-7-Carboxylate](/CAS/GIF/139481-69-9.gif)
139481-69-9
149 suppliers
$8.00/250mg
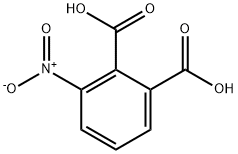
603-11-2
646 suppliers
$15.00/25 g
![Ethyl-2-Ethoxy-1-[[(2'-(1h-Tetrazol-5-Yl)Biphenyl-4-Yl)Methyl]Benzimidazole]-7-Carboxylate](/CAS/GIF/139481-69-9.gif)
139481-69-9
149 suppliers
$8.00/250mg
![BENZOIC ACID, 2-[[(1,1-DIMETHYLETHOXY)CARBONYL]AMINO]-3-NITRO-METHYL ESTER](/CAS/GIF/57113-90-3.gif)
57113-90-3
171 suppliers
$165.00/50mg
![Ethyl-2-Ethoxy-1-[[(2'-(1h-Tetrazol-5-Yl)Biphenyl-4-Yl)Methyl]Benzimidazole]-7-Carboxylate](/CAS/GIF/139481-69-9.gif)
139481-69-9
149 suppliers
$8.00/250mg